Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market is estimated to be valued at USD 154.3 Mn in 2025 and is expected to reach USD 1,610.3 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 39.8% from 2025 to 2032.
Analysts’ Views on Global Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market:
Currently, many key players in the market are looking for a novel treatment for progressive familial intrahepatic cholestasis type 2. This rare genetic disorder affects the liver's ability to transport bile acids, leading to progressive liver damage. Researchers are exploring innovative approaches, such as gene therapy and targeted drug development, to address the underlying cause of the disease and provide effective treatment options for patients. These approaches aim to restore or enhance the liver's ability to transport bile acids, thereby slowing down or halting the progression of liver damage. Additionally, clinical trials are being conducted to evaluate the safety and efficacy of these potential treatments, offering hope for patients and their families.
Figure 1. Global Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market Share (%), By Drug, 2025
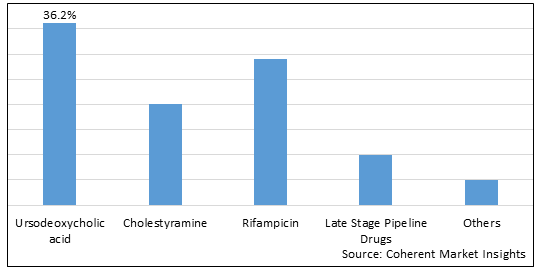
To learn more about this report, Download Free Sample
Global Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market– Drivers
Figure 2. Global Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market Share (%), By Region, 2025
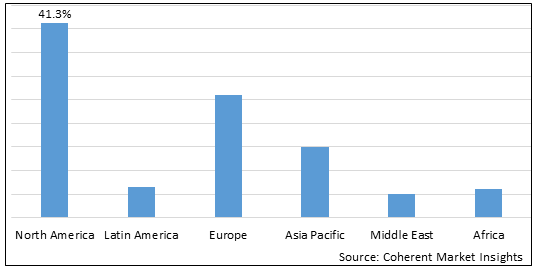
To learn more about this report, Download Free Sample
Global Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market- Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global progressive familial intrahepatic cholestasis type 2 treatment market over the forecast period. North America is estimated to hold 41.3% of the market share in 2025. The global progressive familial intrahepatic cholestasis type 2 treatment market is expected to witness significant growth in the near future, driven by the increasing prevalence of progressive familial intrahepatic cholestasis type 2.
Global Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization (WHO) declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others, faced problems with the transportation of things from one place to another.
Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 154.3 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 39.8% | 2032 Value Projection: | USD 1,610.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Teva Pharmaceutical Industries Ltd., AbbVie, Inc., Glenmark Pharmaceuticals Limited, Par Pharmaceuticals, Inc., Mylan Pharmaceuticals, Inc., Sanofi S.A., Mylan N.V., Novartis International AG, Akorn, Inc., Albireo Pharma, Inc., Mirum Pharmaceuticals, Jadeite Medicines Inc., and Ipsen Pharma. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market Segmentation:
The global progressive familial intrahepatic cholestasis type 2 treatment market is segmented into drug, distribution channel, and region.
Global Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market- Cross Sectional Analysis:
Key players are focusing on presenting clinical trials for the progressive familial intrahepatic cholestasis type 2, which could be expected to boost demand for progressive familial intrahepatic cholestasis type 2 treatment market in the North America region. For instance, on May 17, 2023, Mirum Pharmaceuticals, Inc., a biopharmaceutical company, announced that the company will present data at the upcoming 55th Annual European Society for Pediatric, Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Annual Meeting, taking place in Vienna, Austria. The company will present data from its LIVMARLI (maralixibat) oral solution clinical studies in Alagille syndrome and progressive familial intrahepatic cholestasis (PFIC).
Global Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market: Key Developments
On June 12, 2023, Ipsen, a global, mid-sized biopharmaceutical company, announced that the company will present data from across its growing rare liver disease portfolio at the European Association for the Trial of Liver (EASL) Congress 2023 in Vienna, Austria. These include seven abstracts on new clinical data being presented on Bylvay (odevixibat) when used in patients with progressive familial intrahepatic cholestasis (PFIC) and Alagille syndrome (ALGS). The Bylvay abstracts give more insight into the efficacy and safety profile of the therapy when applied to subgroups of both paediatric and adult PFIC patients. The data report on outcomes includes event-free survival, reduction in serum bile acids (sBAs), pruritus, and other quality of life outcomes in long-term clinical trials and real-world settings.
Global Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market: Key Trends
Global Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market: Restraint
Global Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market - Key Players
Major players operating in the global progressive familial intrahepatic cholestasis type 2 treatment market include Teva Pharmaceutical Industries Ltd., AbbVie, Inc., Glenmark Pharmaceuticals Limited, Par Pharmaceuticals, Inc., Mylan Pharmaceuticals, Inc., Sanofi S.A., Mylan N.V., Novartis International AG, Akorn, Inc., Albireo Pharma, Inc., Mirum Pharmaceuticals, Jadeite Medicines Inc., and Ipsen Pharma.
Definition: Progressive familial intrahepatic cholestasis (PFIC) is a class of chronic cholestasis disorders that comprises a variety of genetic diseases. These conditions begin in infancy and usually progress to cirrhosis within the first decade of life. The average age at onset is 3 months, although some patients do not develop jaundice until later, even as late as adolescence. PFIC can progress rapidly and cause cirrhosis during infancy or may progress relatively slowly with minimal scarring well into adolescence. Few patients have survived into the third decade of life without treatment.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients