An infusion product is a medical device that delivers fluids into a patient's body in controlled amounts such as nutrients and medications. Infusion pumps are commonly used in clinical settings such as hospitals, nursing homes, and home care settings.
An infusion pump is typically operated by a trained user, who programs the rate and duration of fluid delivery via a built-in software interface. Infusion pumps have significant advantages over manual fluid administration including the ability to deliver fluids in very small volumes and at precisely programmed rates or automated intervals.
They can deliver nutrients as well as medications such as insulin or other hormones, antibiotics, chemotherapy drugs, and pain relievers.
U.S. IV Infusion Products Market - Impact of the Coronavirus (COVID-19) Pandemic
Coronavirus (COVID-19) outbreak was first reported on December 31, 2019, in Wuhan, China. The World Health Organization declared COVID-19 as pandemic on March 11, 2020. According to the Coronavirus (COVID-19) Weekly Epidemiological Update by the World Health Organization, over 213 million cases and 4.48 million deaths due to coronavirus disease (COVID-19) were reported till August 25, 2021, across the globe.
Impact of COVID-19 on Demand and Supply of IV Infusion Products
The COVID-19 pandemic and lockdown in various countries across the globe have impacted the financial status of businesses across all sectors including private healthcare sector. The COVID-19 pandemic has impacted the entire supply chain of the healthcare industry mainly due to strict lockdown in several regions. The COVID-19 pandemic has affected the economy of various regions across the globe in three main ways; 1) by directly affecting the production and demand; 2) by creating disruptions in distribution channels; and 3) through its financial impact on companies and financial markets. Many countries such as Thailand, Indonesia, and Singapore are facing problems with regards to transportation and distribution of healthcare products.
However, the coronavirus (COVID-19) pandemic is expected to drive growth of the U.S. IV infusion products market during the forecast period, owing to increasing demand for infusion pumps in COVID-19 patients. For instance, according to Flex Ltd., a multinational electronics contract manufacturer, Singapore, as COVID-19 patients began to fill hospital intensive care units (ICU), infusion pump demand quickly surged. A flood of orders was sent to suppliers, putting pressure on them to manufacture even more. Flex's Timisoara, Romania, facility had been producing infusion pumps for many years, however, in 2020, during the COVID-19 pandemic, the team had to manufacture five times as many infusion pumps, in order to cater to the rising demand.
The U.S. IV infusion products market is estimated to be valued at US$ 7,885.4 Mn in 2021, and is expected to exhibit a CAGR of 3.8% over the forecast period (2021-2028).
Figure 1: U.S. IV Infusion Products Market Share (%) Analysis, By Product Type, 2021
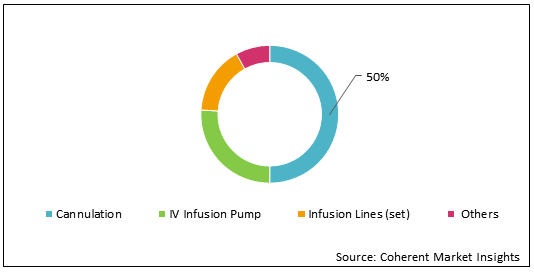
To learn more about this report, Download Free Sample
The increasing prevalence of chronic diseases is the major factor that is expected to drive growth of the U.S. IV infusion products market over the forecast period.
The increasing prevalence of chronic diseases is expected to drive growth of the U.S. IV infusion products market as the IV Infusion products are used for various purposes in chronic diseases such as blood transfusions, chemotherapy, and for administration of intravenous fluids and antibiotic. For instance, according to the article published by the National Health Council, U.S., in 2019, a chronic disease is defined as a disease, which lasts for three months or longer. According to the same source, around, 40 million of the U.S. population are suffering from one or the other chronic disease such as diabetes, heart disease, cancers, chronic lung diseases, stroke, Alzheimer's disease, chronic kidney disease, and others.
U.S. IV Infusion Products Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2020 | Market Size in 2021: | US$ 7,885.4 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2021 to 2028 |
| Forecast Period 2021 to 2028 CAGR: | 3.8% | 2028 Value Projection: | US$ 10,238.5 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
AngioDynamics Inc., Kimal, Terumo Corporation, Teleflex Inc., Becton Dickinson and Company, Cook Group, Nipro Corporation, ZOLL Medical Corporation, B. Braun Medical Inc., AdvaCare Pharma, PL Medical Co.LLC, Greiner Bio-One International GmbH, EMED Technologies Corporation, CODAN Companies, and Smiths Group plc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Certifications from Regulatory Authorities
Increasing certifications from the regulatory authorities for the IV infusion products is expected to provide lucrative growth opportunities for players operating in the U.S. IV infusion products market. For instance, on July 22, 2021, Innovative Health Sciences, LLC, a medical device company focused on developing and commercializing home infusion therapy based in U.S., announced that it received CE Mark Certification for its product, Insignis Syringe Infusion System. Insignis syringe infusion system is the first combination of intravenous and subcutaneous non-electric infusion pump created for use with a selectable rate flow controller, IV controller, and the OneSett.
U.S. IV Infusion Products Market – Restraints
Complications associated with using IV route and IV infusion products is expected to hamper growth of the U.S. IV infusion products market. For instance, according to the article published in the International Journal of Pharmaceutical Studies and Research, 2017, there are many disadvantages associated with administration of drugs through IV route and use of IV products such as pain is felt at the site of injections, extravasation of some drugs can cause injury, necrosis, and sloughing of tissues and severe adverse effect especially when organs such as liver, heart, brain are involved in toxicity.
Figure 2: U.S. IV Infusion Products Market Value (US$ Mn) & Y-o-Y Growth (%), 2020 - 2028
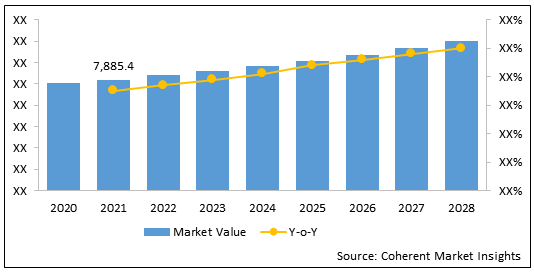
To learn more about this report, Download Free Sample
U.S. IV Infusion Products Market – Competitive Landscape
Major players operating in the U.S. IV infusion products market include AngioDynamics Inc., Kimal, Terumo Corporation, Teleflex Inc., Becton Dickinson and Company, Cook Group, Nipro Corporation, ZOLL Medical Corporation, B. Braun Medical Inc., AdvaCare Pharma, PL Medical Co., LLC, Greiner Bio-One International GmbH, EMED Technologies Corporation, CODAN Companies, and Smiths Group plc.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients