The global anti-epileptic drugs for pediatrics market size is expected to reach US$ 2,533.4 Mn by 2032, from US$ 1,478.2 Mn in 2025, exhibiting a compound annual growth rate (CAGR) of 8.0% during the forecast period.
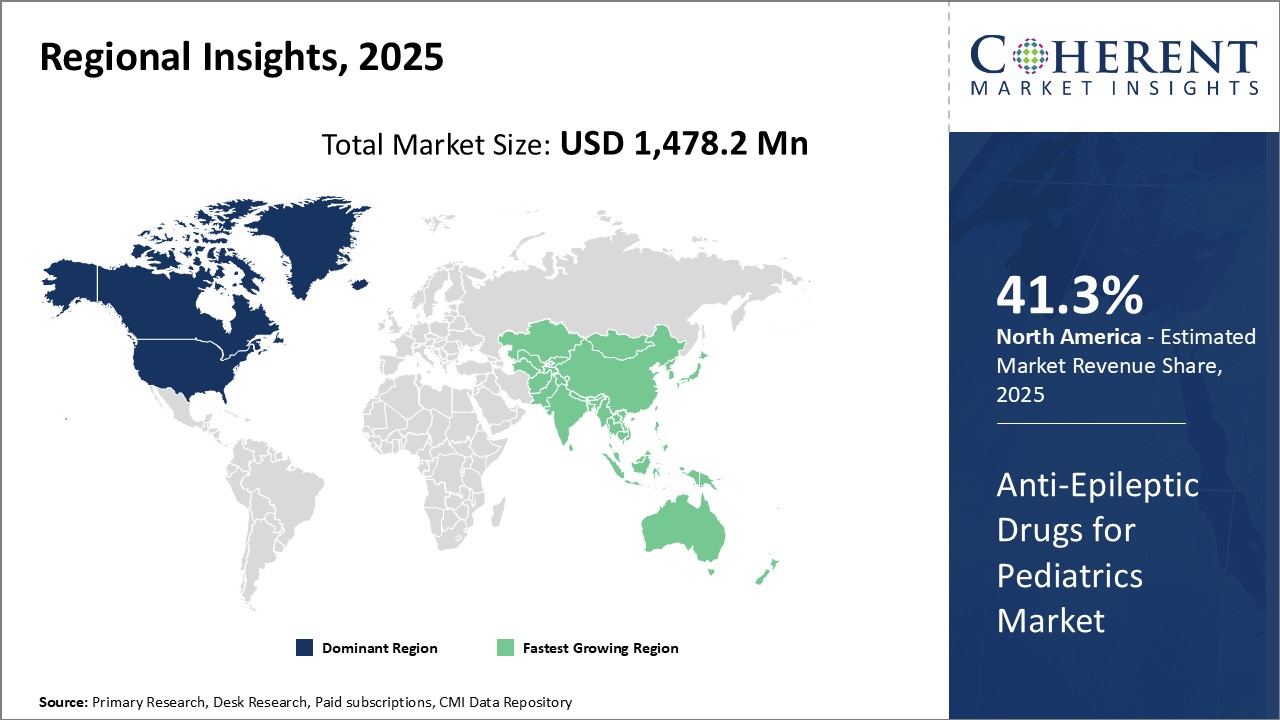
Global Anti-epileptic Drugs for Pediatrics Market Regional Insights
Figure 1. Global Anti-epileptic Drugs for Pediatrics Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Analyst’s Views on the Global Anti-epileptic Drugs for Pediatrics Market:
The global anti-epileptic drugs for pediatrics market is expected to witness steady growth over the forecast period driven by the rising incidence of epilepsy among children worldwide. Moreover, favorable government support through funding for research related to pediatric epilepsy treatment will also propel the market growth. However, strict regulatory guidelines for drug approval and high costs associated with drug development and clinical trials may hamper the market growth to some extent.
Global Anti-epileptic Drugs for Pediatrics Market Drivers
Favorable reimbursement policies
Favorable reimbursement policies across various countries are positively impacting the growth of the global anti-epileptic drugs for pediatrics market for pediatric indications. Governments and healthcare systems worldwide recognize epilepsy as a chronic neurological condition requiring long term treatment. This clinical perspective is translating to supportive reimbursement guidelines that aim to enhance access to quality care and improved outcomes for young patients.
For instance, the Indian government in 2018 updated its National List of Essential Medicines to include newer anti-seizure medications specifically for use in children. This helped reduce financial barriers for families by making these vital drugs available at affordable costs through public health facilities. Similarly, many European countries like U.K., Germany, and France have reimbursement schemes either through national health insurers or social security systems to cover part or full expenses of anti-epileptic prescriptions for the pediatric population.
Global Anti-epileptic Drugs for Pediatrics Market Restraints
Global Anti-epileptic Drugs For Pediatrics Market Opportunities
Untapped emerging markets: Untapped emerging markets could indeed present a major opportunity for growth in the global anti-epileptic drugs for pediatrics market. Many developing nations still have limited access to effective treatments for pediatric epilepsy due to various economic and infrastructure challenges. However, these nations are experiencing rapid development and expansion of their healthcare systems.
Investing in customized distribution and affordability programs for emerging markets could be key to capitalizing on this opportunity. Some adaptations may be required to package and deliver anti-epileptic drugs in ways that make them accessible even in remote communities with underdeveloped medical infrastructure and purchasing power limitations. Partnerships with local health authorities and not-for-profit organization (NGOs) working in these nations can help gain important insights into the socio-economic landscape as well as distribution challenges. This collaborative approach will be vital to ensure steady supply of effective anti-epileptic treatment options reach every child in need, thereby unlocking the growth prospects in these dynamic but underserved markets.
Rising demand for targeted therapy: The increasing demand for personalized treatment options is having a significant impact on the global anti-epileptic drugs market for pediatric market. With advancements in genetic research and molecular diagnostics targeted therapies that are designed for certain genetic subtypes or biomarkers of epilepsy are becoming increasingly prevalent. For example, some of the newer anti-epileptic drugs in the market, such as Fintepla, have been formulated for the treatment of seizures associated with specific genetic mutations related to Dravet syndrome and Lennox-Gastaut syndrome. These targeted medications promise higher efficacy for children with treatment-resistant forms of epilepsy caused by these underlying genetic traits. The success of such precision treatments has driven both patients and doctors to demand more customized therapeutic alternatives.
Anti-Epileptic Drugs for Pediatrics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,478.2 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.0% | 2032 Value Projection: | USD 2,533.4 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Sanofi, Eisai Co., Ltd., Pfizer Inc., UCB S.A., Sunovion Pharmaceuticals Inc., Abbott, Jazz Pharmaceuticals plc, Ovid Therapeutics Inc., SK BIOPHARMACEUTICALS, Alexza Pharmaceuticals, Inc., Taisho Pharmaceutical Holdings Co., Ltd., Marinus Pharmaceuticals, Inc., Xenon Pharmaceuticals Inc., H. Lundbeck A/S, and Teva Pharmaceutical Industries Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Anti-epileptic Drugs For Pediatrics Market Trends
Increasing investments by key market players
Increasing adoption of inorganic growth strategies, such as investments, is expected to drive the global anti-epileptic drugs for pediatrics market over the forecast period.
For instance, in March 2022, Aculys Pharma, Inc., a pharmaceutical company, announced that it has raised a total of US$ 24 million in Series B Round with the participation by JIC Venture Growth Investments, Mitsubishi UFJ Capital, and Spiral Capital as new investors. The funding helps for clinical development and commercial activities for launching research and development activities of epileptic seizure emergencies
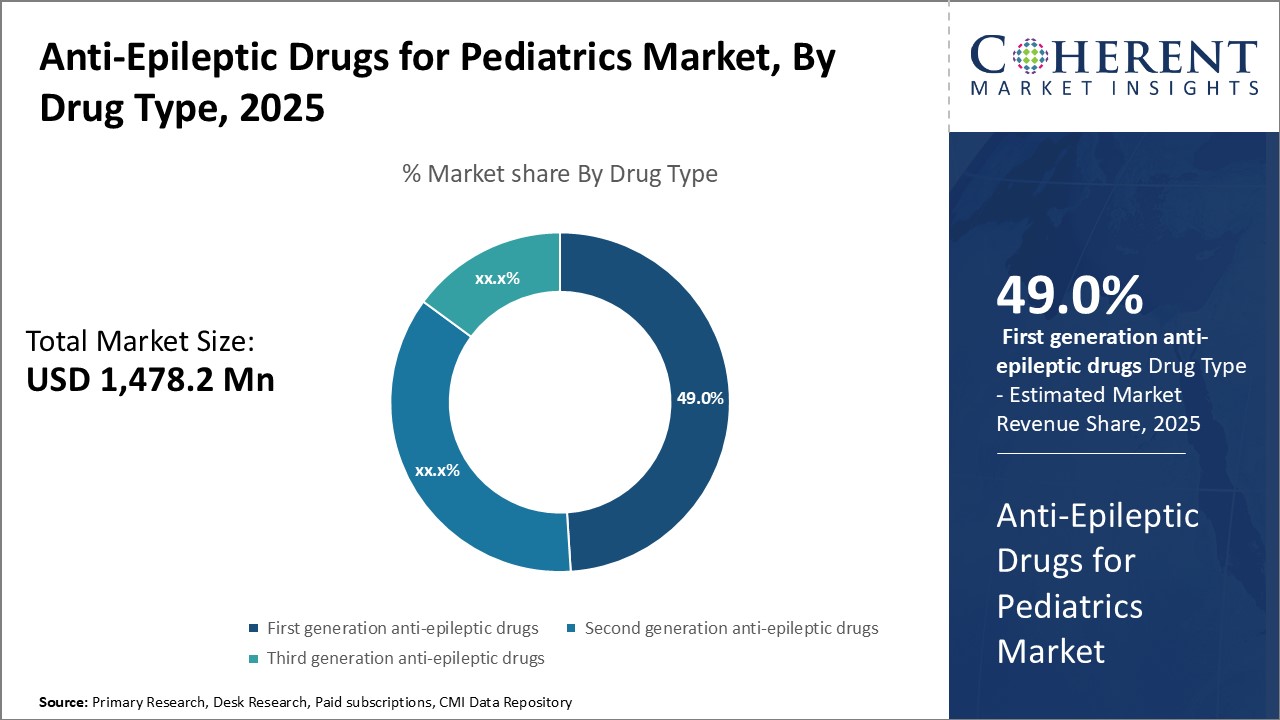
Figure 2. Anti-Epileptic Drugs for Pediatrics Market Share (%), By Drug Type, 2025
To learn more about this report, Download Free Sample
Recent Developments
Product Approvals
Acquisitions and agreements
Top Companies in the Global Anti-epileptic Drugs For Pediatrics Market
Definition: Epilepsy is associated with disrupted activities in the brain called seizures, which affects the central nervous system. Depending on the area of the brain obstructed by seizures, these are categorized into generalized seizures and partial seizures. Generalized seizures affect the whole brain, while partial seizures affect just one part of the brain. Depending on their severity, seizures are termed as mild seizures and stronger seizures. Mild seizures are difficult to diagnose, as these last for only a few seconds. Stronger seizures may last for a few seconds to several minutes, resulting in spasms and uncontrollable muscle twitches. This may cause the patient to lose consciousness, and experience temporary loss of cognition, or memory loss.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients