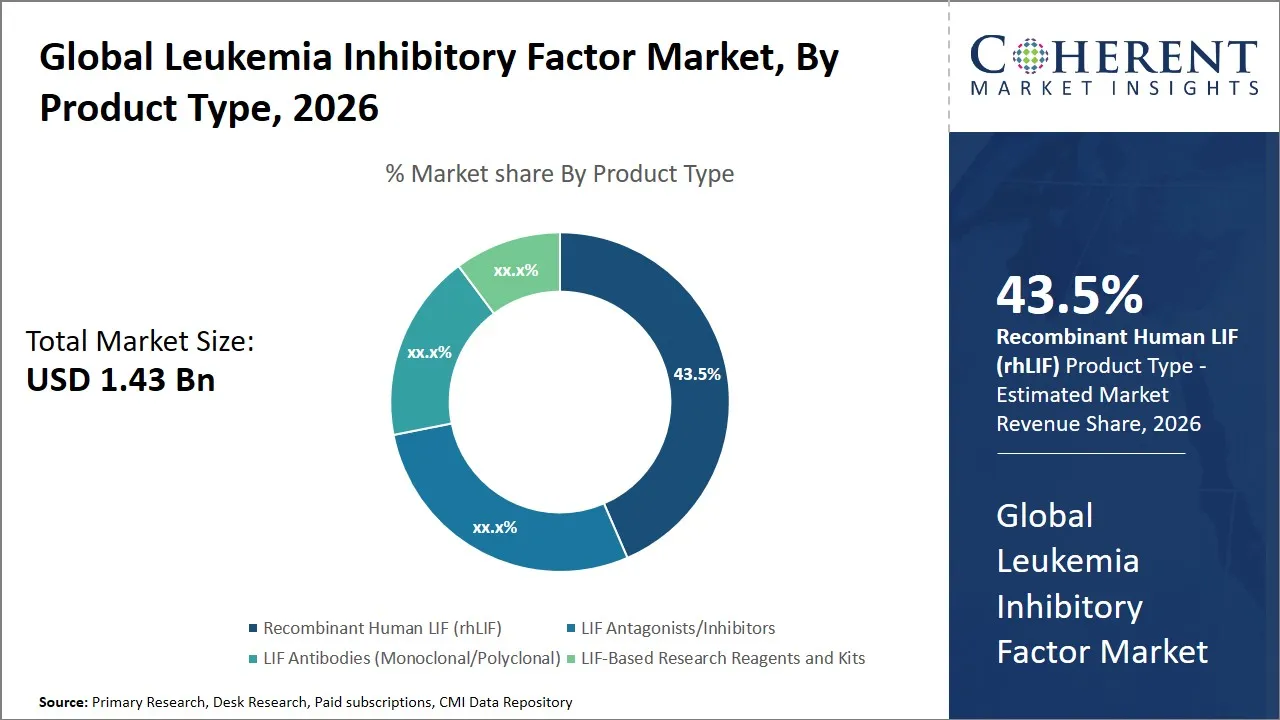
Global Leukemia Inhibitory Factor Market Size and Forecast – 2026 to 2033
According to Coherent Market Insights, the global leukemia inhibitory factor market is estimated to be valued at USD 1.43 Bn in 2026 and is expected to reach USD 2.65 Bn by 2033, exhibiting a compound annual growth rate (CAGR) of 9.2% from 2026 to 2033. This significant growth reflects the increasing demand for novel therapeutic solutions and advancements in biotechnology focused on leukemia therapeutics, positioning the market as a promising sector for investment and research over the forecast period.
Key Takeaways of the Leukemia Inhibitory Factor Market
- In 2026, Recombinant Human LIF (rhLIF) segment is expected to dominate the global market, holding a share of 43.5%.
- Among the different source segments, leukemia inhibitory factor derived from mammalian cells is expected to lead with a share of 32.5% in 2026.
- Within the application segment, research and development is the largest sub-segment, accounting for an estimated 57.9% of the market share in 2026.
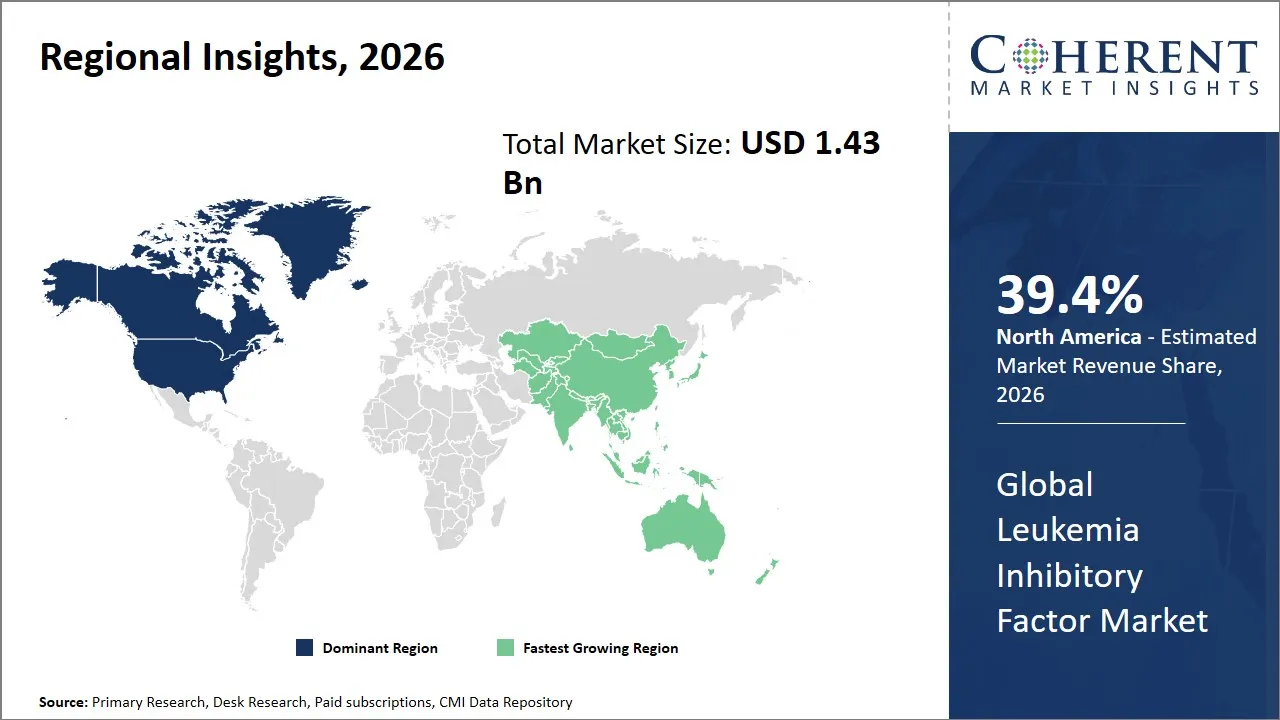
- North America is expected to lead the market, holding a share of 39.4% in 2026. Asia Pacific is anticipated to be the fastest-growing region, with 25.4% share in 2026.
Market Overview
- Market trends highlight a growing emphasis on personalized medicine and targeted therapies leveraging Leukemia Inhibitory Factor (LIF) due to its potential in managing hematological malignancies and regenerative medicine applications.
- Innovations in drug delivery systems, combined with rising research funding and collaborations between pharmaceutical companies and research institutions, are driving clinical developments.
- Additionally, rising awareness and improved diagnostic capabilities are accelerating the adoption of LIF-based therapies, further fueling the market expansion.
Currents Events and Its Impacts
|
Current Events |
Description and its Impact |
|
Rapid Integration of AI in Drug Discovery & Biomarker Identification |
|
|
Expansion of Biosimilars and Biologics in Emerging Markets |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Why is Recombinant Human LIF (rhLIF) Dominating the Leukemia Inhibitory Factor Market?
In terms of product type, Recombinant Human LIF (rhLIF)is expected to hold a predominant share of the global market with 43.5% in 2026, primarily because of its broad range of applications and superior biological activity compared to other LIF products. The recombinant form of LIF, produced using modern biotechnological methods, offers highly purified and consistent protein quality, which is critical for both research and therapeutic purposes.
This attribute is a key factor fueling the demand for rhLIF, as it enables more reliable and reproducible results in biological assays and clinical investigations. Furthermore, the versatility of rhLIF in various research paradigms, including stem cell maintenance and differentiation, cancer therapeutics, and immunomodulation studies, significantly boosts its adoption.
For example, Proteintech Group, Inc. expanded the availability of recombinant Leukemia Inhibitory Factor for stem cell research. The endotoxin-free LIF protein supports pluripotent stem cell maintenance and leukemia cell assays. Everon Life Sciences is enabling local access for Indian research laboratories.
How is Mammalian Cell–Derived LIF Driving Growth in the Leukemia Inhibitory Factor Market?
Among various source segments, mammalian cell-derived segment is projected to command the highest share with 32.5% in 2026 driven largely by its superior structural and functional fidelity in replicating native human LIF. Mammalian expression systems, such as Chinese Hamster Ovary (CHO) or HEK293 cells, offer complex post-translational modifications including glycosylation patterns that are crucial for the biological activity and stability of LIF proteins.
This closer resemblance to natural human LIF enhances the molecule’s therapeutic relevance and efficacy in experimental setups, which is why researchers and clinicians display a strong preference for this source. The increasing demand for biologics that demonstrate high specificity and minimal immunogenicity is a pivotal factor supporting growth in the mammalian cell–derived segment.
How is Research and Development Fueling the Expansion of the Leukemia Inhibitory Factor Market?
Within the application spectrum, research and development (R&D) is the leading segment with an estimated share of 57.9% in 2026, propelled largely by the persistent global focus on understanding the biological functions of LIF and its implications across multiple fields of life sciences.
The molecule’s involvement in vital biological processes such as cell differentiation, immune regulation, and embryonic development drives extensive fundamental research, thereby ensuring sustained demand for LIF products in academic and industrial laboratories.
Clinical-Grade vs Research-Grade Leukemia Inhibitory Factor (LIF) Pricing & Margin Gap
|
Dimension |
Research‑Grade LIF |
Clinical‑Grade LIF |
Pricing & Margin Gap |
|
Price (per mg) |
US$300 – US$1,000 |
US$5,000 – US$25,000+ |
Clinical 5×–25× higher |
|
Production Standard |
Non-GMP |
GMP Certified |
GMP adds significant cost |
|
Manufacturing Cost |
Low–Moderate |
High |
Clinical cost 3×–8× higher |
|
Regulatory Compliance |
Minimal |
Substantial (GMP) |
Regulatory compliance drives gap |
|
Gross Margin |
40%–60% |
60%–85% |
Clinical grade has higher margin |
|
Target Buyers |
Academia, R&D |
Biotech, Pharma, Clinical |
Clinical less price-sensitive |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Regional Insights
To learn more about this report, Download Free Sample
North America Leukemia Inhibitory Factor Market Analysis and Trends
North America’s dominance in the global leukemia inhibitory factor market with an estimated 39.4% share in 2026 is driven by a mature healthcare infrastructure, high prevalence of leukemia and associated disorders, and significant investments in biotechnology and pharmaceutical research. The U.S., as a leader in biomedical innovation, hosts numerous prominent biotech companies and research institutions focusing on regenerative medicine and oncology, fostering strong R&D activities around LIF applications.
Favorable government policies promoting the development of novel therapeutics, including expedited review processes by agencies like the U.S. FDA, create an enabling environment for rapid product commercialization. The region’s well-established ecosystem, comprising contract research organizations (CROs), academic collaborations, and pharmaceutical giants, facilitates robust clinical trials and product launches.
Asia Pacific Leukemia Inhibitory Factor Market Analysis and Trends
Asia Pacific is expected to exhibit the fastest growth in the leukemia inhibitory factor market with 25.4% in 2026, driven by increasing healthcare expenditure, rising prevalence of hematologic disorders, and expanding biotech industry ecosystems across key countries such as China, Japan, South Korea, and India. Government initiatives aimed at fostering innovation in biopharmaceuticals, alongside improving healthcare access, accelerate the adoption of advanced therapies.
The increasing presence of multinational corporations alongside emerging domestic biotech firms fuels competitive developments in the region. Notable players like Takeda Pharmaceutical (Japan), Biocon (India), and Fosun Pharma (China) play pivotal roles in product development, manufacturing, and distribution, thereby enhancing market penetration.
Global Leukemia Inhibitory Factor Market Outlook for Key Countries
What are the Key Trends Shaping the U.S. Leukemia Inhibitory Factor Market?
The U.S. leukemia inhibitory factor market is characterized by strong industry-academia partnerships and substantial investment in cutting-edge biotechnology research. Institutions like the National Institutes of Health provide significant funding that supports advanced studies on LIF’s therapeutic applications in leukemia and other immune-related disorders.
Leading companies such as Amgen and Gilead Sciences are active in developing LIF-targeted treatments, leveraging sophisticated clinical trial networks and regulatory pathways. The prevalence of precision medicine initiatives further supports the integration of LIF-based interventions into personalized treatment protocols.
Is Germany the Next Growth Engine for the Leukemia Inhibitory Factor Market?
Germany maintains a prominent position within Europe’s leukemia inhibitory factor market, supported by robust healthcare infrastructure and a proactive regulatory framework that encourages biosimilar development and biologics innovation. The country’s strong pharmaceutical sector includes key players like Bayer and BioNTech, which contribute to oncology and regenerative medicine research.
German research institutions frequently collaborate with global pharmaceutical firms in advancing LIF therapeutic platforms. Additionally, government incentives for biotechnology innovation enhance market growth prospects by enabling efficient translation of research into clinical applications.
Japan Leukemia Inhibitory Factor Market Trends
Japan’s leukemia inhibitory factor market is distinguished by its advanced research ecosystem and substantial government support for regenerative medicine and immunotherapy development under programs such as the Japan Agency for Medical Research and Development (AMED). Companies like Takeda Pharmaceutical and Chugai Pharmaceutical actively develop and commercialize novel LIF-related therapies.
The country’s aging population with a rising incidence of hematological disorders drives demand for innovative treatments. Japan’s efficient regulatory pathways for orphan drugs and regenerative therapies further facilitate accelerated product approvals and market entry.
China Leukemia Inhibitory Factor Market Trends
China’s rapidly expanding biotech market underpins its position as a critical growth hub for LIF therapeutics. Government policies such as “Made in China 2025” and substantial funding for biotechnology innovation foster a competitive landscape. The rise of domestic pharmaceutical companies, including Fosun Pharma and Shanghai Junshi Biosciences, enhances indigenous capability in both research and manufacturing.
Additionally, increasing clinical trial activity and broader healthcare access stimulate the market growth. Collaboration between foreign biotech firms and Chinese companies also accelerates technology transfer and local product development.
India Leukemia Inhibitory Factor Market Trends
India’s leukemia inhibitory factor market benefits from an expanding biopharmaceutical industry supported by increasing healthcare infrastructure and a large patient pool. Government initiatives like the Biotechnology Industry Research Assistance Council (BIRAC) provide critical funding and support for innovation in biologics, including LIF-based therapies.
Companies such as Biocon and Serum Institute of India are instrumental in developing and manufacturing biosimilar and novel biologics targeting hematologic malignancies. Moreover, India’s growing focus on affordable healthcare solutions drives the demand for cost-effective LIF therapeutics, making it a vital component of the global market landscape.
Cell Therapy & Regenerative Medicine Dependency for Leukemia Inhibitory Factor Market
- Leukemia Inhibitory Factor (LIF) plays a crucial role in cell therapy and regenerative medicine due to its involvement in stem cell maintenance, differentiation, and survival. Its ability to regulate pluripotent stem cells makes it a valuable biomolecule for therapeutic applications, including tissue regeneration and the treatment of degenerative diseases. As regenerative medicine advances, LIF's role in creating favorable microenvironments for stem cells will drive its demand for clinical-grade use, particularly in applications like cellular reprogramming and immune modulation.
- The dependency on LIF in cell therapy lies in its potential to enhance cell-based therapies, particularly in the fields of oncology and tissue engineering. Researchers are leveraging LIF to develop treatments that promote cell proliferation and differentiation, improving the success rates of regenerative therapies. With growing clinical trials exploring LIF's therapeutic potential, the market for LIF is poised to expand, underpinned by its importance in fostering the growth of next-generation regenerative medicine solutions.
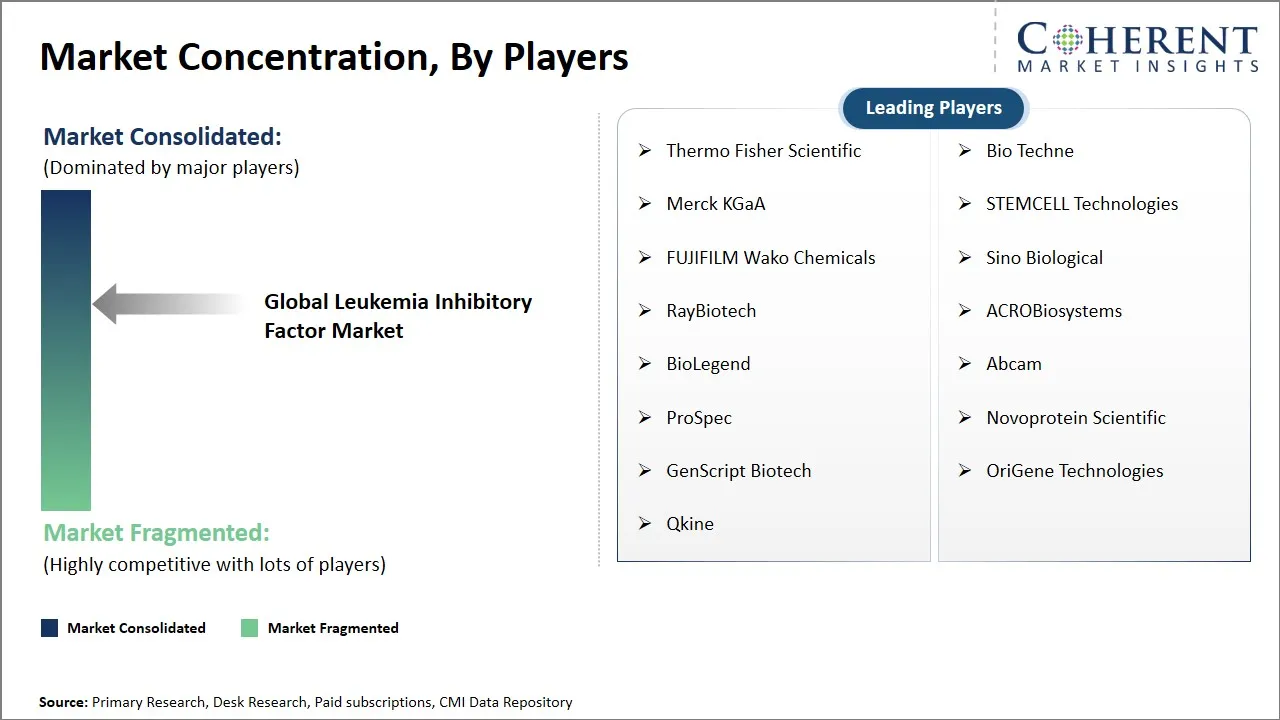
Market Players, Key Developments, and Competitive Intelligence
To learn more about this report, Download Free Sample
Key Developments
- In September 2025, Eli Lilly and Company announced positive Phase 3 results for Jaypirca in CLL and SLL. The drug significantly improved progression free survival in treatment naïve patients versus chemoimmunotherapy. Results strengthen confidence in non-covalent BTK inhibitors alongside broader Leukemia Inhibitory Factor oncology research. Lilly plans regulatory submissions to expand Jaypirca into earlier leukemia treatment settings.
- In August 2025, Dizal Pharmaceutical received U.S. FDA Fast Track Designation for birelentinib in relapsed CLL and SLL. The first-in-class dual LYN and BTK inhibitor showed an 84.2% response rate in heavily pretreated patients. The decision highlights unmet needs in leukemia treatment alongside emerging Leukemia Inhibitory Factor research relevance. Dizal plans accelerated global development to advance birelentinib for resistant B-cell malignancies.
- In December 2025, Bristol Myers Squibb secured U.S. FDA approval for Breyanzi in relapsed CLL and SLL. Breyanzi became the first CAR T cell therapy approved for this difficult leukemia setting. Clinical data showed durable responses, addressing major unmet needs beyond BTK and BCL-2 inhibitors. The approval advances cell therapy innovation alongside evolving Leukemia Inhibitory Factor driven oncology research.
- In September 2023, GSK received U.S. FDA approval for Ojjaara in myelofibrosis patients with anemia. Ojjaara became the first approved therapy addressing anemia, splenomegaly, and symptoms regardless of prior treatment. The decision highlights progress in hematologic innovation alongside broader Leukemia Inhibitory Factor pathway research relevance. The approval establishes a new treatment option for a high unmet need blood cancer population.
Top Strategies Followed by Global Leukemia Inhibitory Factor Market Players
|
Player Type |
Strategic Focus |
Examples |
|
Established Market Leaders |
Heavy R&D investment in advanced formulations and delivery mechanisms; strategic alliances with OEMs and research institutions; aggressive geographic expansion into emerging markets like Asia Pacific, Latin America, and the Middle East |
Genmab focuses on biologic therapies and expanding presence in emerging markets; Bristol-Myers Squibb aggressively invests in R&D for next-generation immunotherapies and targets Asia Pacific growth. |
|
Mid-Level Players |
Emphasis on cost-effective and reliable products; optimizing manufacturing processes; strategic collaborations with larger firms, licensing agreements, and joint ventures to expand production capabilities and market reach |
Novartis develops cost-effective biosimilars and has joint ventures in emerging regions; Amgen expands through licensing agreements and regional collaborations. |
|
Small-Scale Players |
Concentration on specialized features or innovative product offerings; adoption of cutting-edge bioengineering and novel delivery systems; local partnerships for market penetration |
Argenx focuses on innovative immunotherapy products for niche indications; Immunomedics partners with academic institutions to develop novel therapies for targeted treatments. |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Report Scope
Leukemia Inhibitory Factor Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 1.43 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 9.2% | 2033 Value Projection: | USD 2.65 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Thermo Fisher Scientific, Bio Techne, Merck KGaA, STEMCELL Technologies, FUJIFILM Wako Chemicals, Sino Biological, RayBiotech, ACROBiosystems, BioLegend, Abcam, ProSpec, Novoprotein Scientific, GenScript Biotech, OriGene Technologies, and Qkine |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
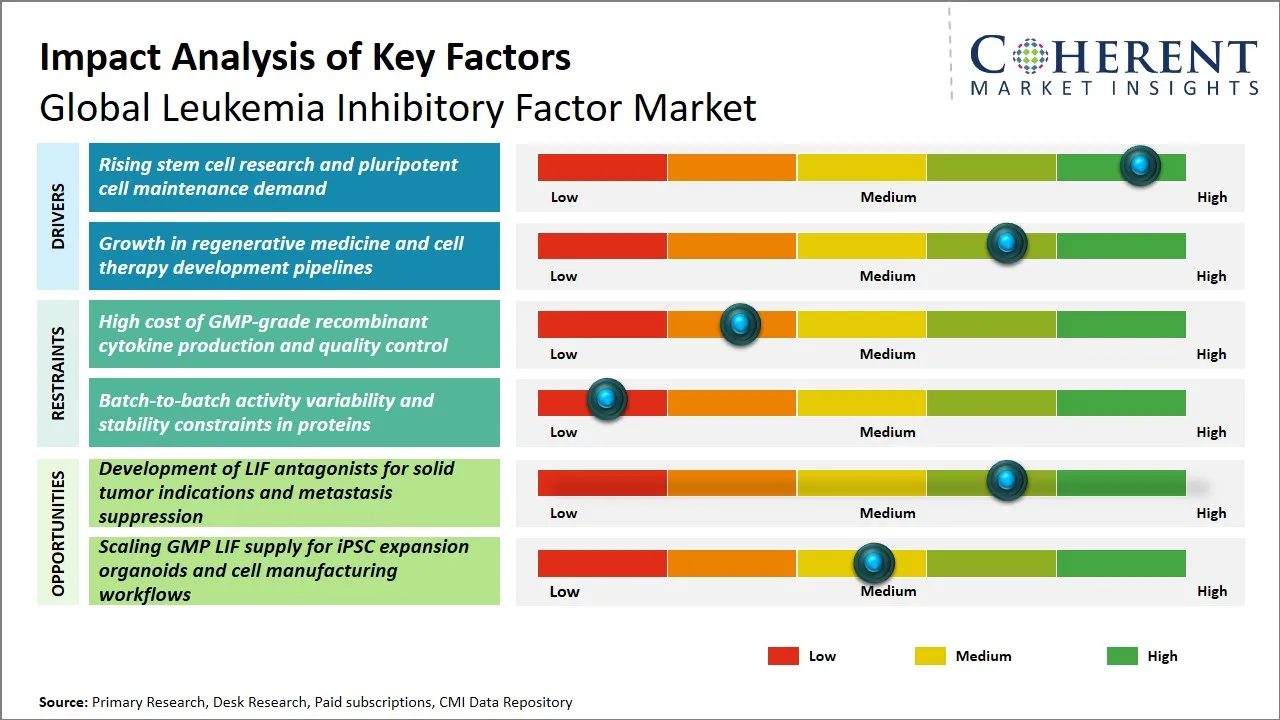
Leukemia Inhibitory Factor Market Dynamics
To learn more about this report, Download Free Sample
Leukemia Inhibitory Factor Market Driver - Rising stem cell research and pluripotent cell maintenance demand
The escalating focus on stem cell research and the advancement of pluripotent cell technologies are key factors propelling the demand for leukemia inhibitory factor (LIF) in the life sciences sector. LIF plays a critical role in maintaining the self-renewal and pluripotency of embryonic stem cells, which are essential for developing regenerative therapies and personalized medicine. As researchers strive to unlock new therapeutic applications for degenerative diseases and genetic disorders, the ability to reliably culture and sustain pluripotent stem cells in vitro becomes increasingly vital.
In February 2025, STEMCELL Technologies highlighted advancements in pluripotent stem cell expansion for scalable immunotherapy. Corning Life Sciences showcased defined, xeno-free surfaces enabling consistent PSC growth for clinical use. Xcell Biosciences demonstrated bioreactor systems improving hypoxic expansion efficiency. These innovations support cell therapies aligned with emerging Leukemia Inhibitory Factor research applications.
Leukemia Inhibitory Factor Market Opportunity - Development of LIF Antagonists for Solid Tumor Indications and Metastasis Suppression
The development of Leukemia Inhibitory Factor (LIF) antagonists for solid tumor indications and metastasis suppression represents a significant growth opportunity within the global leukemia inhibitory factor market. Historically, LIF has been primarily associated with hematological malignancies; however, emerging research highlights its pivotal role in the tumor microenvironment of various solid cancers, including breast, pancreatic, and ovarian tumors. By modulating LIF signaling pathways, LIF antagonists have the potential to inhibit tumor progression, reduce metastatic spread, and overcome resistance to conventional therapies. This expands the therapeutic scope of LIF beyond traditional blood cancers, addressing a considerable unmet clinical need in solid tumor management.
For example, Genmab is investigating LIF-related signaling pathways for new oncology therapies. Amgen is focused on monoclonal antibodies targeting LIF to reduce metastasis, especially in pancreatic and breast cancers. Merck & Co. is researching LIF antagonists to overcome treatment resistance in solid tumors like ovarian cancer. Bristol-Myers Squibb is exploring LIF antagonism in combination with immuno-oncology therapies to enhance tumor suppression.
Analyst Opinion (Expert Opinion)
- The leukemia inhibitory factor market is growing rapidly due to advancements in molecular biology and drug delivery systems. Increasing focus on LIF’s role in solid tumors like breast, pancreatic, and ovarian cancers is opening new therapeutic possibilities. Regulatory support and rising demand for innovative treatments are fueling investments, but challenges such as overcoming resistance to current therapies remain.
- Global events like the AACR Annual Meeting and ESMO Congress have played a key role in advancing LIF research. Companies such as Genmab, Amgen, and Merck & Co. are leading with promising LIF-targeted therapies, including monoclonal antibodies that reduce metastasis. These efforts are expected to drive the market growth and improve outcomes for solid tumor patients.
Market Segmentation
- Product Type Insights (Revenue, USD Bn, 2021 - 2033)
- Recombinant Human LIF (rhLIF)
- LIF Antagonists/Inhibitors
- LIF Antibodies (Monoclonal/Polyclonal)
- LIF-Based Research Reagents and Kits
- Source Insights (Revenue, USD Bn, 2021 - 2033)
- Mammalian Cell–Derived
- Bacterial (E. coli)–Derived
- Yeast-Derived
- Synthetic/Engineered Variants
- Application Insights (Revenue, USD Bn, 2021 - 2033)
- Research and Development
- Stem Cell Biology and Reprogramming
- Cancer Research
- Immunology and Inflammation Studies
- Neurology Research
- Clinical and Therapeutic Development
- In vitro Fertilization (IVF) and Embryology
- Drug Discovery and Biomarker Development
- Research and Development
- End User Insights (Revenue, USD Bn, 2021 - 2033)
- Academic and Research Institutes
- Pharmaceutical and Biotechnology Companies
- Contract Research Organizations (CROs)
- Clinical Research Centers
- Diagnostic and Testing Laboratories
- Regional Insights (Revenue, USD Bn, 2021 - 2033)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Thermo Fisher Scientific
- Bio Techne
- Merck KGaA
- STEMCELL Technologies
- FUJIFILM Wako Chemicals
- Sino Biological
- RayBiotech
- ACROBiosystems
- BioLegend
- Abcam
- ProSpec
- Novoprotein Scientific
- GenScript Biotech
- OriGene Technologies
- Qkine
Sources
Primary Research Interviews
- Industry Stakeholders List
- Oncologists
- Biotech Company Executives
- End-users List
- Hospitals
- Pharmaceutical Distributors
Government and International Databases
- U.S. Food and Drug Administration (FDA)
- European Medicines Agency (EMA)
- National Institutes of Health (NIH)
- World Health Organization (WHO)
- Centers for Disease Control and Prevention (CDC)
- National Cancer Institute (NCI)
Trade Publications
- Pharmaceutical Technology
- BioPharma Reporter
- The Cancer Letter
- Drug Development & Delivery
- Journal of Clinical Oncology
- Pharmaceutical Business Review
Academic Journals
- The Journal of Leukocyte Biology
- Clinical Cancer Research
- Nature Reviews Drug Discovery
- The Lancet Oncology
- Journal of Immunotherapy
- Journal of Hematology & Oncology
Reputable Newspapers
- The New York Times
- The Guardian
- The Wall Street Journal
- Reuters Health
- The Washington Post
- BBC News
Industry Associations
- American Association for Cancer Research (AACR)
- European Society for Medical Oncology (ESMO)
- American Society of Clinical Oncology (ASCO)
- International Society for Stem Cell Research (ISSCR)
- Global Biotech Alliance
- Biotechnology Innovation Organization (BIO)
Public Domain Resources
- ClinicalTrials.gov
- National Cancer Institute (NCI) Database
- European Bioinformatics Institute (EBI)
- OpenFDA
- United Nations Office on Drugs and Crime (UNODC)
Proprietary Elements
- CMI Data Analytics Tool: Proprietary analytics tool to analyze real-time market trends, consumer behavior, and technology adoption in market
- Proprietary CMI Existing Repository of Information for Last 8 Years
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients