The Protein stability analysis Market size is valued US$ 2.44 Billion in 2025 and is expected to reach US$ 5.23 Billion by 2032, growing at a compound annual growth rate (CAGR) of 11.55% from 2025 to 2032. Protein stability analysis involves studying the conditions under which a protein can retain its functional three-dimensional structure. This analysis is important for applications that are related to protein engineering, drug discovery, and formulation development. There are multiple techniques that are used to assess protein stability.
Protein Stability Analysis Market Regional Insights:
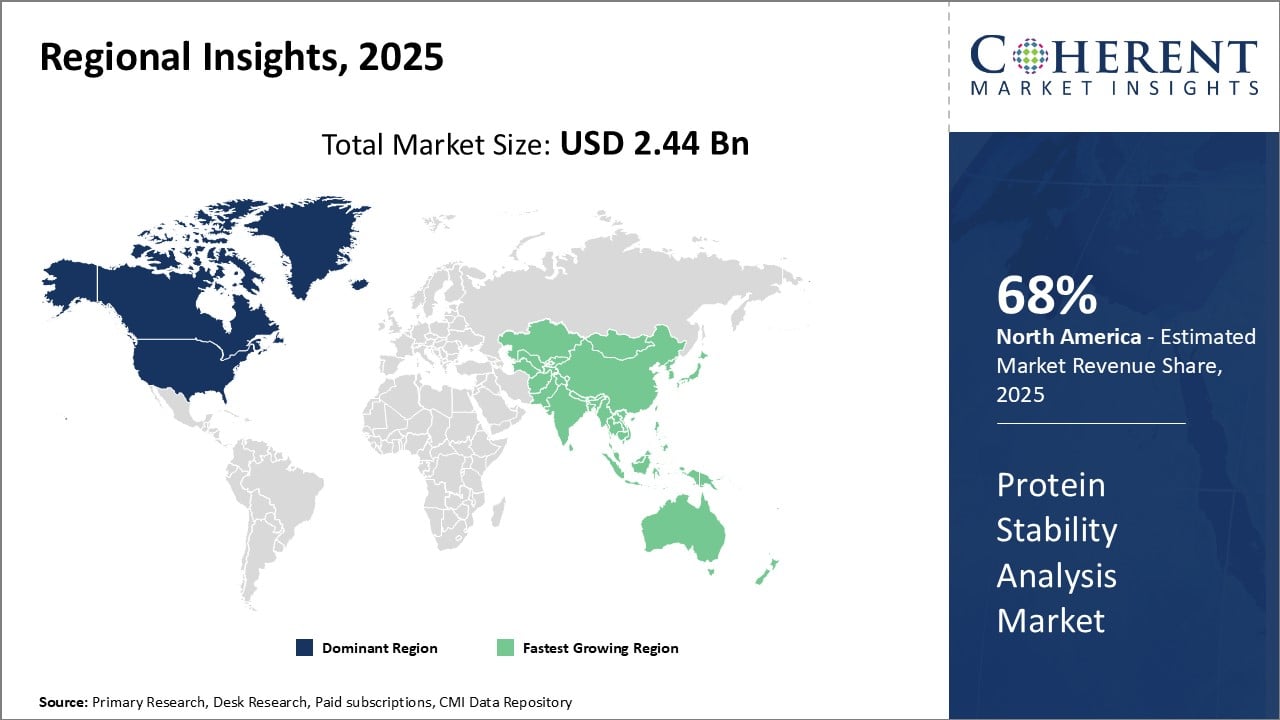
- North America: It is well established that the market for protein stability analysis is dominated by North America. There is a considerable need for protein characterization and stability testing instruments in the region, where top pharmaceutical and biotechnology businesses, comprising 68% of the market, are largely concentrated in nations like the U.S. and Canada. The North American offices of several leading CMOs and CROs engaged in biological drug development and drug discovery encourage widespread use of protein stability analysis tools. In addition, the area is a leader in this field's technological breakthroughs. North America is home to several major suppliers of tools, supplies, and software for protein stability analysis. They make significant research and development (R&D) investments to bring forward novel solutions, which support the preservation of their technological lead. The use of these analytical solutions is also mandated by strict requirements pertaining to the characterization of active pharmaceutical components and stability testing of biological medicines.
- Asia Pacific: The market for protein stability analysis is expanding at the quickest rate in the Asia Pacific area. This is explained by the growth of pharmaceutical production capacities in several Asian nations, including South Korea, China, and India. To take advantage of cost benefits, many producers of biosimilars, generic drugs, and new biotech companies locate their manufacturing facilities in these nations. As a result, there is a growing need in Asia Pacific for upstream analytical tools such as assays and devices for protein stability analysis.
- Europe: It is projected that the European market for protein stability analysis would grow rapidly. Growing interest in personalized medicine and the need for protein therapeutics are driving the business. The U.K., Germany, and France are the three largest markets in Europe for protein stability study. U.K. has the largest market in the region due to the large number of pharmaceutical companies and academic research centers located there. Further factors driving the need for protein stability studies include the growing use of protein treatments in the treatment of various diseases and the growing demand for biosimilars.
Figure 1. Protein Stability Analysis Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Analyst Viewpoint:
The protein stability analysis market is expected to witness significant growth in the future. The market is primarily driven by rising R&D investments in pharmaceutical and biotechnology industries. Increasing focus on developing highly effective biologics and growing demand for quality control and standardization of protein formulations will further support the market expansion. However, stringent regulatory guidelines for new product approvals may moderately restraint the market during the forecast period.
North America, especially the U.S., will continue dominating the protein stability analysis market supported by presence of major pharmaceutical companies and growing biotechnology research in the region. Asia Pacific is likely to be the fastest growing market due to increasing pharmaceutical production and improving healthcare infrastructure in various Asia Pacific (APAC) countries. Europe will also provide lucrative opportunities for market players owing to rising government funding for pharmaceutical R&D projects.
Within the protein stability analysis platform segment, chromatography systems are most widely used. However, spectrophotometers will witness higher adoption in the near future. Serum albumin and monoclonal antibodies will remain the most common protein types analyzed for stability. Outsourced testing services are expected gain more popularity among small pharmaceutical companies with limited in-house capabilities. Market players operating in the protein stability analysis market should focus on developing integrated platforms with advanced software for enhanced productivity and cost-savings. This will help them expand in emerging Asia markets effectively.
Protein Stability Analysis Market Drivers:
- Growing demand from pharmaceutical industry: The demand for protein stability analysis from the pharmaceutical industry is growing rapidly as protein-based drugs witness increased adoption. Proteins are large and complex molecules that need to retain their 3D structures which are known as conformations, to function as intended in the human body. Even small changes to a protein's conformation can reduce its effectiveness or cause unwanted side effects. Therefore, pharmaceutical companies rely heavily on protein stability analysis to effectively research, develop, and manufacture new protein-based drugs such as antibodies, enzymes and vaccines. Protein stability analysis helps evaluate proteins under a wide range of conditions like varying temperatures, solvents, mechanical agitation, and light exposures to determine if they maintain intended conformations. This provides valuable insights into optimal drug formulation, storage, distribution and administration conditions. For example, stability analysis helps to develop a stable formulation for a vaccine against a novel strain of coronavirus. For instance, in October 2021, according to the data provided by the National Institute of Health nearly 4000 biologic drugs targeting over 700 distinct protein targets are in development.
- Adoption in biopharmaceutical manufacturing: Adoption of biopharmaceutical manufacturing has been significantly driving the growth of protein stability analysis over the past years. As biologics have become the primary focus of drug development pipelines, thereby ensuring the stability of protein therapeutics throughout development and production which has become a major area of focus. Protein stability testing helps biopharmaceutical manufacturers thoroughly characterize their protein drugs, improving safety, and efficacy. Most biologic drug candidates face instability issues such as aggregation, degradation, and fragmentation during development and manufacturing. Comprehensive stability analysis by using techniques such as fluorescence spectroscopy, differential scanning calorimetry, and chromatography enables developers to better understand a protein's stability profile under different stresses such as temperature, agitation, and freeze-thaw cycles. This allows for modifications to be made to formulations, storage conditions and processes to maximize the shelf-life of the therapeutic. As the biopharmaceutical industry moves towards advanced modalities like antibody-drug conjugates, biosimilars, and gene therapies, which have additional complexities, thus the need for robust stability assessment has increased significantly.
- For instance, according to the World Health Organization (WHO), over 3,300 biotherapeutic products, including vaccines, were in development globally till 2021.
Protein Stability Analysis Market Opportunities:
- Adoption of multi-omics approach in protein analysis: Adoption of multi-omics approach in protein analysis holds great promise to revolutionize the protein stability analysis market. The integration of genomics, transcriptomics, proteomics, and other 'omics' techniques allows for a comprehensive understanding of protein structure and interactions within the cellular environment. This holistic approach provides valuable insights into protein folding, modifications and degradation pathways that cannot be gleaned through single 'omics' analysis. It helps researchers develop a system-level perspective that are required to elucidate complex mechanisms governing protein stability. For instance, according to the article "Proteomics in the pharmaceutical and biotechnology industry: a look into the future" published by the Journal of Proteomics in 2021, the collection of large-scale proteomic, genomic, proteomic, and lipidomic datasets offers the opportunity to combine these data modalities and build a more comprehensive understanding of biological systems. The article also states that the trend of combining functional genomic and proteomic datasets is picking up pace among biotech and pharmaceuticals industries.
- Advancements in screening technologies: Advancements in screening technologies present a major opportunity for growth in the protein stability analysis market. Next-generation platforms that enable high-throughput screening of protein formulations are enabling researchers to analyze protein stability with enhanced efficiency, speed, and resolution as compared to traditional assays. Technologies such as differential scanning fluorimetry, which allows real-time monitoring of protein unfolding upon thermal stress, have evolved significantly. Newer fluorescence-based devices incorporate microplates and nanoliter sample volumes, thereby facilitating automated screening of hundreds of conditions simultaneously. This high-throughput capability is the key to address the rising complexity of protein therapeutics and reagents. For instance, according to the article "Applications of synchrotron powder X-ray diffractometry in drug development" published in the International Journal of Pharmaceutics in 2021, synchrotron-based analytical methods have accelerated monoclonal antibody formulation development from 18-24 months to less than 12 months.
Protein Stability Analysis Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.44 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.55% | 2032 Value Projection: | USD 5.23 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Thermo Fisher Scientific, Inc., Enzo Biochem, Inc., PerkinElmer Inc., NanoTemper, GE Healthcare, HORIBA, Ltd., Malvern Panalytical Ltd., Agilent Technologies, Inc., SETARAM Instrumentation, Unchained Labs, Waters Corporation, and Applied Photophysics Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Protein Stability Analysis Market Trends:
- Automation and miniaturization of protein analysis workflow: The protein analysis market is undergoing significant changes due to the growing trend of automation and miniaturization of workflows. Advancements in microfluidics and lab-on-a-chip technologies are enabling automation and miniaturization of the entire protein analysis process from sample preparation to separation, detection, and data analysis on a single microfluidic chip that is optimized to handle nanoliter volumes of protein samples. This addresses many challenges that is faced by biopharmaceutical companies with traditional technologies such as requirement of large sample volumes, complex and time-consuming multi-step manual processes and need for specialized skilled workforce, thereby improving efficiency and reducing turnaround times drastically. For instance, according to the Nanotemper Technologies website, the nanoDSF system by using premium tip technology can analyze up to 384 samples continuously with an ultra-low sample consumption of just 3 μL per well . NanoTemper Technologies GmbH is a company that manufactures scientific monolith instruments for biomolecular analysis.
- Shift towards label-free protein analysis techniques: The protein stability analysis market is witnessing significant changes as label-free techniques for protein analysis are gaining more prominence. With label-free methods, proteins do not require labelling with radioactive elements, fluorescent tags or other modifications for their detection. This enables analysis of proteins in their native states without altering their structures and functions. More researchers and pharmaceutical companies prefer label-free technologies such as microscale thermophoresis, bio-layer interferometry, and surface plasmon resonance over traditional methods as they provide a reliable, rapid and reagent-free way to study protein stability, interactions and conformational changes. For example, use of microscale thermophoresis is growing in the drug discovery sector to screen drug candidates for binding affinities with target proteins and to calculate binding parameters. This technique has successfully aided in finding hit compounds for various disease targets over the years according to recent reports provided by the National Institutes of Health.
Protein Stability Analysis Market Restraints:
- High capital investment requirements: The high capital investment requirements for instruments and equipment needed for protein stability analysis is one of the major factors restraining the growth of this market. Setting up a lab for protein stability analysis from scratch requires very expensive investments in advanced analytical instruments such as differential scanning calorimeters (DSC), fluorescence spectrometers, chromatographic systems, and others. These instruments can cost anywhere between US$50,000 to US$500,000 based on their type and capabilities. Moreover, the running costs of maintaining such sophisticated analysis equipment and consumables that are required for various assays are also significant. This massive upfront capital crunch poses difficulties for small and mid-sized research organizations as well as startups working on protein-based drug development and other related areas.
- Counterbalance: Researchers can apply for grants from governmental, non-profit, and private organizations. This funding can help cover the costs of purchasing advanced equipment or accessing shared facilities.
- Limitations in conventional protein stability analysis techniques: Limitations of conventional protein stability analysis techniques are a major factor driving the growth of the protein stability analysis market. Traditional methods such as Differential Scanning Calorimetry (DSC) and dynamic light scattering provide useful structural insights but have limitations when it comes to throughput, cost-effectiveness and translation to high volume applications. DSC, for instance, requires milligrams of protein sample and cannot be used for screening purposes involving multiple variants. This has led researchers and industry to look for modern techniques that overcome these issues. Novel surface plasma resonance-based sensors and microfluidic-chip assays now allow high sensitivity stability measurements using picogram levels of protein in a miniaturized format suitable for parallel analyses. For instance, according to the European Commission's 2020 report on research infrastructure, over 200 European facilities have invested in new technologies aimed at applications in biologic drug development, food sciences, and environmental monitoring.
- Counterbalance: Implementing cutting-edge analytical tools such as mass spectrometry, nuclear magnetic resonance (NMR) spectroscopy, and X-ray crystallography can provide more detailed insights into protein stability and structure than traditional methods.
Recent Developments:
- In 2021, Agilent Technologies Inc. announced a definitive agreement to acquire Resolution Bioscience Inc., a leader in developing and commercializing next-generation sequencing (NGS)-based precision oncology solutions. The acquisition was completed, and it was intended to expand Agilent Technologies Inc.'s precision medicine offerings and complement its capabilities in NGS-based cancer diagnostics.
- Agilent Technologies Inc is a global company headquartered in Santa Clara, California, that provides instruments, software, services, and consumables for laboratories.
- The company was established in 1999 as a spin-off from Hewlett-Packard and has since grown to become a leading provider of scientific solutions for various industries, including chemical analysis, life sciences, and diagnostics . Agilent Technologies offers a wide range of products and services.
- In 2021, Thermo Fisher Scientific, Inc. launched a new protein stability analysis kit called the protein thermal shift 96 Kit. The kit is designed to measure thermal stability and identify protein candidates for drug discovery and development. This launch is expected to increase the company's market share in the protein stability analysis market.
- Thermo Fisher Scientific Inc. is an American supplier of analytical instruments, life sciences solutions, specialty diagnostics, laboratory, pharmaceutical, and biotechnology services . The company was formed in 2006 through the merger of Thermo Electron and Fisher Scientific. Thermo Fisher Scientific is headquartered in Waltham, Massachusetts, and has a global presence, with over 130,000 employees worldwide . The company's products are sold under the brand names of Thermo Scientific, Fisher Scientific, and several other recognized brand names.
- In 2020, PerkinElmer, Inc. launch its new LabChip GXII touch protein system. The system is designed to provide automated protein analysis, characterization, and quality control. This launch is expected to increase the company's market share in the protein stability analysis market. PerkinElmer, Inc. is a global corporation that provides technology and service solutions for various industries, including diagnostics, life science research, food, environmental and industrial testing . The company was founded in 1937 and has evolved from focusing on precision optics to offering a wide range of capabilities, including detection, imaging, informatics, and service. PerkinElmer's products and services encompass analytical instruments, lab technologies, diagnostic testing, laboratory services, informatics, and cord blood banking .
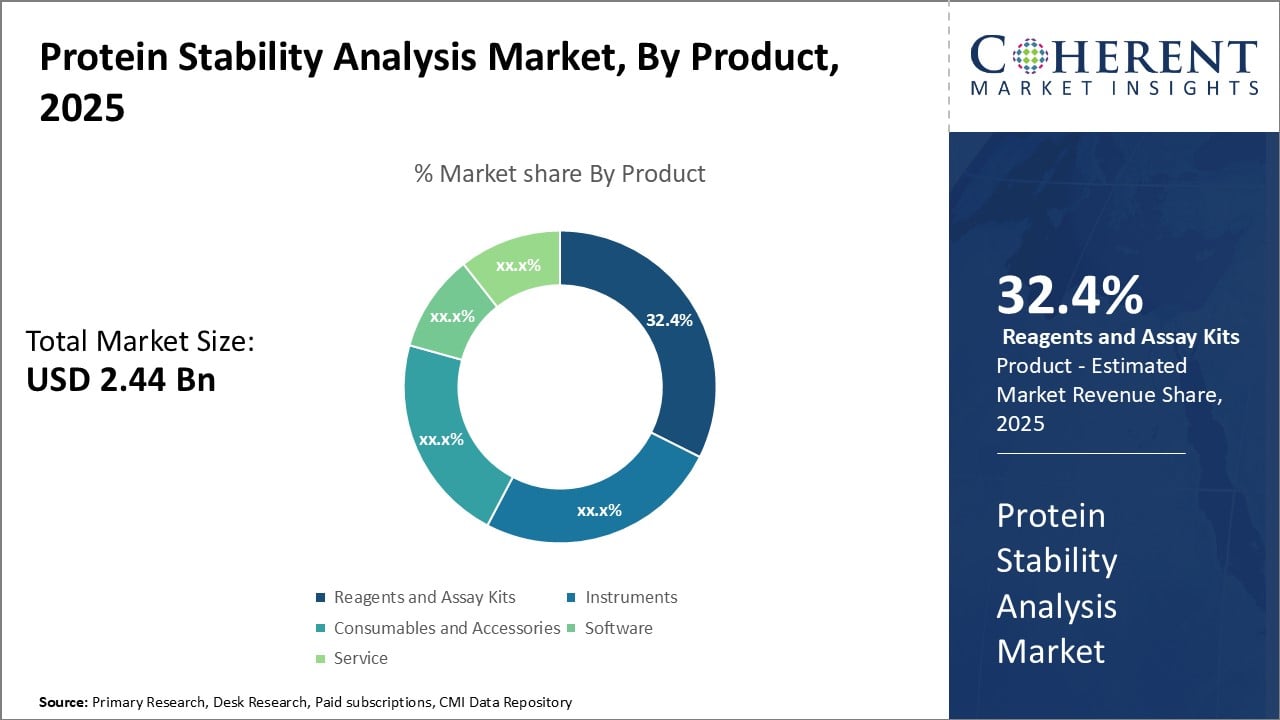
Figure 2. Protein Stability Analysis Market Share (%), By Product, 2025
To learn more about this report, Download Free Sample
Top Key Players:
- Thermo Fisher Scientific, Inc.
- Enzo Biochem, Inc.
- PerkinElmer Inc.
- NanoTemper,
- GE Healthcare
- HORIBA, Ltd.
- Malvern Panalytical Ltd.,
- Agilent Technologies, Inc.
- SETARAM Instrumentation
- Unchained Labs,
- Waters Corporation
- Applied Photophysics Ltd.
Definition: Protein stability analysis one of the fundamental assays that are performed in the research laboratories and by the pharmaceutical companies. As a result, the protein stability analysis market is on the rise. Pharmaceutical companies are constantly searching for new drugs and as a result, the demand for protein stability analysis techniques is on the increase. Research institutes are playing a major role in the discovery of new diseases and new therapies. As a result of increased government funding, the market for protein stability analysis is also on the rise.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




