Achondroplasia Treatment Market is estimated to be valued at USD 238.5 Mn in 2025 and is expected to reach USD 2,105.8 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 36.5% from 2025 to 2032.
Analysts’ Views on Global Achondroplasia Treatment Market:
The global market for achondroplasia treatments is anticipated to grow over the forecast period due to increasing awareness of the importance of diagnosis of congenital illnesses. In addition, an before increase in achondroplasia cases prompts key players and research institutions to conduct more clinical trials for the development of novel treatments. The global market growth is anticipated to be driven by an increase in investments in R&D for the management and treatment of achondroplasia during the forecast period. Moreover, the global achondroplasia treatment market is anticipated to grow as a result of the approval of new treatments for this condition.
Figure 1. Global Achondroplasia Treatment Market Share (%),By Treatment Type, 2025
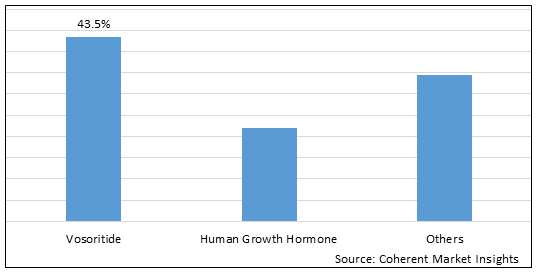
To learn more about this report, Download Free Sample
Global Achondroplasia Treatment Market– Driver
Figure 2. Global Achondroplasia Treatment Market Share(%) by Region, 2025
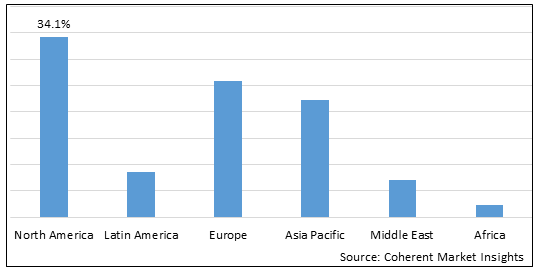
To learn more about this report, Download Free Sample
Global Achondroplasia Treatment Market- Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global achondroplasia treatment market over the forecast period. North America is estimated to hold 34.1% of the market share in 2025. The global achondroplasia treatment market is expected to witness significant growth in the coming years, driven by the high prevalence of achondroplasia, favorable health reimbursement, and increased awareness. North America is expected to emerge as the leading region for the achondroplasia treatment market. For instance, in April 2022, the University of Arkansas sponsored a clinical trial to characterize skeletal muscle amino acid kinetics to an Extrinsic allergic alveolitis EAA challenge, i.e., an oral amino acid tolerance test (OATT), to determine the state of muscle health. For that purpose, trial subjects were given 10 g of a commercially available nutritional supplement for achondroplasia. Hence, an increase in such clinical studies demonstrating the clinical outcome of supplements to treat achondroplasia may result in the launch of new supplements and thus contribute to the growth of the studied market in the region. These kinds of developments in the healthcare system and rising health expenditures, as well as increased knowledge of advanced cancer treatments, are fueling the achondroplasia treatment market growth in North America.
Global Achondroplasia Treatment Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others, were facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a positive impact on the global achondroplasia treatment market. The COVID-19 outbreak affected the market's growth adversely in its preliminary phase; however, the number of cancer patients increased in Pandemic, and so has the demand for the achondroplasia market. which is expected to hamper the global achondroplasia treatment market over the forecast period, owing to delayed clinical trials of products. For instance, the annual report of Ascendis Pharma A/S 2020, a biopharmaceutical company, stated that due to the pandemic, there was a potential impact on the conduct of clinical trials of investigational drug candidates.
Achondroplasia Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 238.5 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 36.5% | 2032 Value Projection: | USD 2,105.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
BioMarin, RIBOMIC, Ascendis Pharma A/S, BridgeBio Pharma, Inc., Pfizer Inc., PhaseBio Pharmaceuticals, Inc., SiSaf, Novo Nordisk A/S, F. Hoffmann-La Roche Ltd, LG Chem, Ferring B.V., JCR Pharmaceuticals Co., Ltd, KVK TECH, INC., VIVUS LLC., ProLynx Inc., Teva Pharmaceutical Industries Ltd., Eli Lilly and Company, Ipsen Pharma, Novartis AG, and Xiamen Amoytop Biotech Co., Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Achondroplasia Treatment Market Segmentation:
The global achondroplasia treatment market report is segmented into Treatment Type, Route of Administration, Distribution Channel, and Region.
Among all the segmentations, the treatment type segment is expected to dominate the market over the forecast period, and this is attributed to the increased awareness of achondroplasia is expected to boost the growth of achondroplasia treatment market over the forecast period.
Global Achondroplasia Treatment Market Cross Sectional Analysis:
Various government initiatives support research and development of achondroplasia treatments, further contributing to market growth. For instance, according to the article published in February 2022 in PubMed, the U.S. Department of Health and Human Services was one of the leading funding organizations that sponsored 1,604 sarcopenia-related articles. The U.S. is one of the top countries in the North American region that contributes more toward achondroplasia-related research and development. This increased research is anticipated to lead to various innovations in the country, driving the growth of the achondroplasia treatment market in the country.
Global Achondroplasia Treatment Market: Key Developments
On March 7, 2023, BioMarin Pharmaceutical Inc., a biotechnology company dedicated to transforming lives through genetic discovery, announced that the U.S. Food and Drug Administration (FDA) had accepted the company's supplemental New Drug Application (sNDA) for VOXZOGO (vosoritide) for injection to expand treatment in the U.S. to include children with achondroplasia under the age of 5. Achondroplasia is the most common form of disproportionate short stature.
In August 2020, Ascendis Pharma A/S, a biopharmaceutical company that utilizes its innovative TransCon technologies to address unmet medical needs, announced that the European Commission (EC) had granted Orphan Designation to TransCon C-Type Natriuretic Peptide (CNP) for the treatment of achondroplasia, the most common form of dwarfism. TransCon CNP is an investigational long-acting prodrug of CNP designed to provide the continuous exposure of CNP at safe, therapeutic levels via a single, weekly subcutaneous dose. TransCon CNP also received in 2020, orphan designation for the treatment of achondroplasia in the U.S.
On June 1, 2023, Myriad Genetics, Inc., a company of genetic testing and precision medicine, launched new studies and expansion of its Precise Oncology Solutions portfolio at the 2023 American Society of Clinical Oncology (ASCO).
In January 2020, Wacker Biotech is technological leader in the chemical industry and manufactures products for all key global industries. The company Provides Active Ingredient to treat achondroplasia in children global phase-2 Trial for Ascendis Pharma is a biopharmaceutical company that focuses on developing drugs for endocrinology and oncology indications.
Global Achondroplasia Treatment Market: Key Trends
Global Achondroplasia Treatment Market: Restraint
Global Achondroplasia Treatment Market- Key Players
Major players operating in the global achondroplasia treatment market include BioMarin, RIBOMIC, Ascendis Pharma A/S, BridgeBio Pharma, Inc., Pfizer Inc., PhaseBio Pharmaceuticals, Inc., SiSaf, Novo Nordisk A/S, F. Hoffmann-La Roche Ltd, LG Chem, Ferring B.V., JCR Pharmaceuticals Co., Ltd, KVK TECH, INC., VIVUS LLC., ProLynx Inc., Teva Pharmaceutical Industries Ltd., Eli Lilly and Company, Ipsen Pharma, Novartis AG, and Xiamen Amoytop Biotech Co., Ltd.
Global Achondroplasia Treatment Market– Definition: Achondroplasia is a genetic disorder that affects the fibroblast growth factor receptor protein in the body. This protein begins to function abnormally in achondroplasia, slowing bone development in the growth plate cartilage. This results in shorter bones, abnormally shaped bones, and a shorter stature; adults with achondroplasia range in height from 42 to 56 inches. The genetic defect can be passed from parent to child. However, in approximately 80% of instances, achondroplasia is caused by a spontaneous mutation (a sudden genetic defect) that originates in the developing embryo.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients