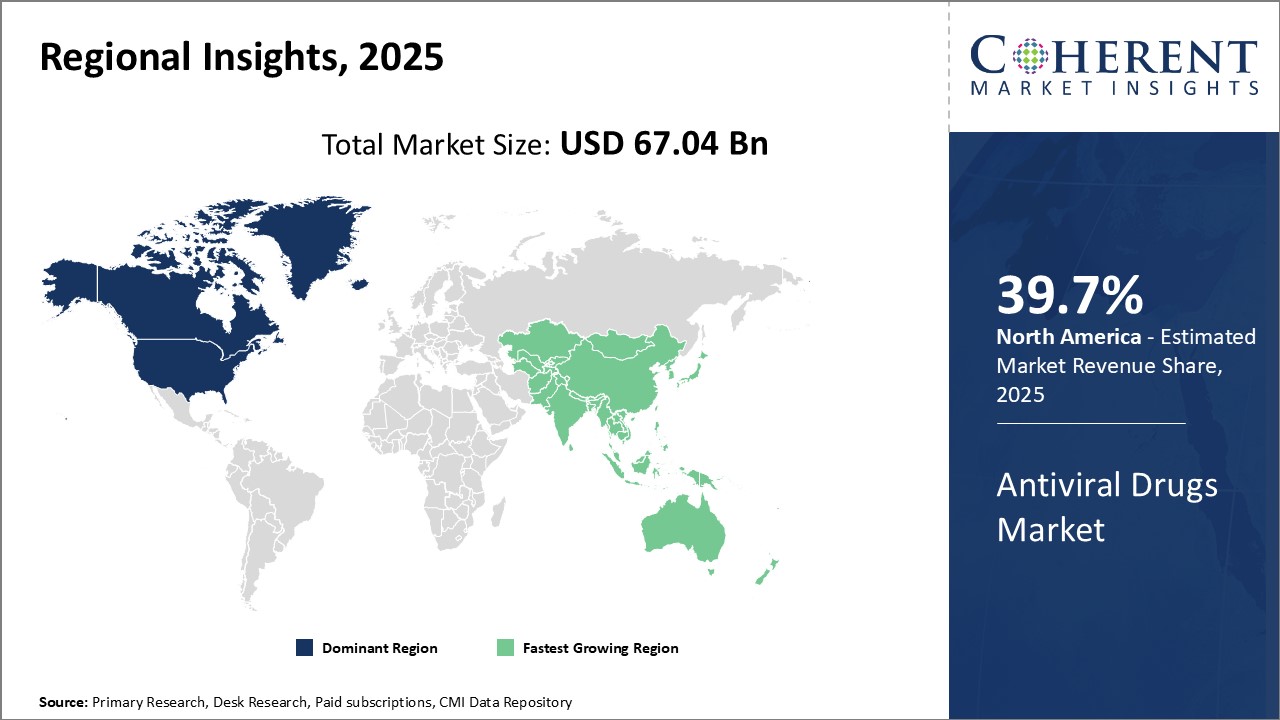
Global antiviral drugs market is estimated to be valued at USD 67.04 Bn in 2025 and is expected to reach USD 96.30 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 5.3% from 2025 to 2032.
To learn more about this report, Download Free Sample
The global Antiviral Drugs Market is witnessing robust growth, driven by the increasing prevalence of viral infections and heightened awareness of early treatment options. DNA polymerase inhibitors lead the market due to their proven effectiveness, while branded drugs dominate owing to better accessibility and physician preference. Hospital pharmacies remain the key distribution channel, ensuring reliable access to critical care medications.
|
Current Event |
Description and its impact |
|
Resurgence of Global Viral Outbreaks |
|
|
Advancements in Antiviral Drug Formulations |
|
|
Patent Expiry and Rise of Generics |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The antiviral drugs market is witnessing a robust pipeline of novel therapies aimed at addressing both existing and emerging viral threats. Key pharmaceutical companies are investing heavily in research and development to create next-generation antivirals with improved efficacy, broader spectrum, and lower resistance rates.
Prominent candidates in late-stage clinical trials target high-priority infections such as HIV, hepatitis B and C, respiratory syncytial virus (RSV), and cytomegalovirus. Additionally, efforts are underway to develop broad-spectrum antivirals capable of responding rapidly to pandemic threats like novel influenza strains and coronaviruses.
Advances in RNA interference (RNAi) technology, CRISPR-based antivirals, and immunomodulators are also shaping the future landscape. Strategic collaborations between biotech firms, governments, and global health organizations are accelerating the development timeline. With a growing focus on unmet clinical needs and drug resistance mitigation, the antiviral pipeline holds significant potential to transform treatment paradigms and expand market opportunities over the next decade.
The antiviral drugs market features a dynamic and competitive patent landscape, driven by continuous innovation and the urgent need to combat evolving viral infections. Leading pharmaceutical companies such as Gilead Sciences, Merck & Co., GlaxoSmithKline, and AbbVie dominate global patent filings, focusing on novel drug formulations, combination therapies, and targeted mechanisms of action.
A substantial number of patents are centred on nucleotide and nucleoside analogs, protease inhibitors, and polymerase inhibitors, which are pivotal in treating HIV, hepatitis, and influenza. Recent trends show increased filings in RNA-based antivirals and CRISPR-derived therapies, reflecting the market's shift toward precision medicine and pandemic preparedness.
Additionally, patent extensions and exclusivity rights for blockbuster antivirals like remdesivir and tenofovir highlight the strategic use of intellectual property to sustain market dominance. As generic manufacturers aim to enter post-patent expiration segments, the evolving IP landscape is set to influence pricing, innovation, and global accessibility of antiviral therapies.
In 2025, reimbursement for antiviral drugs remains a pivotal factor shaping market access and affordability. In North America, comprehensive insurance coverage through Medicare, Medicaid, and private payers ensures broad access to both branded and generic antivirals, particularly for chronic conditions like HIV and hepatitis.
Value-based contracting and rebate mechanisms are increasingly used to manage costs while ensuring patient access to innovative therapies. Many public and private payers require step therapy or prior authorization, especially for high-cost, novel antivirals.
In Europe, national health systems and regional payers employ health technology assessments (HTAs) to evaluate cost-effectiveness and negotiate pricing. Drugs demonstrating significant clinical benefit, such as long-acting injectable antivirals or improved bioavailability formulations, often secure favourable reimbursement and tenders. However, budget constraints can delay reimbursement decisions, impacting launch and access in certain markets.
In emerging economies, including parts of Asia-Pacific and Latin America, government reimbursement schemes are gradually expanding to include critical antivirals under universal health coverage plans. Tiered pricing, donor-funded programs, and public–private partnerships play essential roles in supporting equitable access across socio-economic groups.
In the antiviral drugs market, prescribers prioritize efficacy, safety profile, and patient adherence when selecting treatment options. Branded antiviral drugs remain the preferred choice among healthcare providers, especially in critical care and hospital settings, due to their proven clinical performance, consistent supply, and comprehensive patient support programs.
Physicians tend to favour antivirals with a broad spectrum of activity, fewer side effects, and simplified dosing regimens, which enhance treatment compliance particularly important for chronic conditions like HIV and hepatitis.
Prescribers also lean towards drugs with strong real-world evidence and those recommended in established clinical guidelines. For instance, long-acting formulations or fixed-dose combinations that reduce pill burden are increasingly favoured. However, in cost-sensitive settings or where insurance coverage is limited, generics are gaining acceptance, provided they meet regulatory quality standards. Ultimately, prescribers’ preferences are shaped by a balance of clinical outcomes, regulatory approval, and accessibility for diverse patient populations.
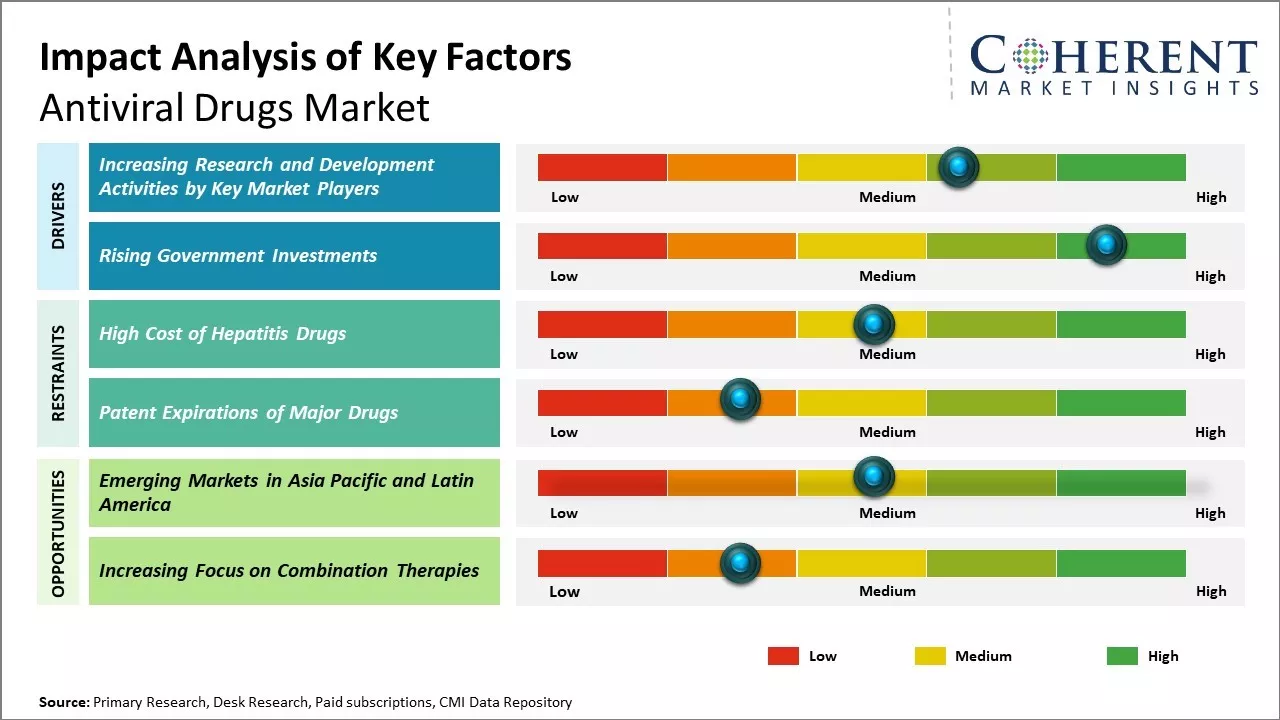
Increasing research and development activities by key market players is expected to drive the market growth over the forecast market. For instance, in March 2021, Pfizer, Inc., a pharmaceutical company, announced the initiation of Phase 1 study in healthy adults to evaluate the safety and tolerability of an investigational, novel oral antiviral therapeutic for SARS-CoV-2, the virus.
Rising government investments in key companies to promote research and development for the treatment of viral infection is expected to offer a lucrative opportunity for the market players to develop novel therapeutics for the treatment of viral infection. For instance, in July 2020, Novavax (a biotechnology company) and Regeneron Pharmaceuticals, a biotechnology company, received US$ 2 billion funding from the U.S. federal government to manufacture drugs and vaccines.
Increasing focus on combination therapies is indeed a great opportunity for growth in the global antiviral drugs market. As researchers gain a deeper understanding of viral biology and mechanisms of drug resistance, combination therapies are seen as a more effective clinical strategy compared to monotherapies.
Instead of targeting a single viral protein or mechanism, combinations can act on multiple viral lifecycle stages or pathways simultaneously. This makes the development of resistance much more challenging for the virus.
The DNA polymerase inhibitors segment is projected to dominate the global antiviral drugs market with a 36.7% share in 2025, driven by its broad-spectrum effectiveness against numerous viral infections.
These inhibitors obstruct the function of DNA polymerase—an enzyme critical for viral replication—making them especially effective against conditions such as herpes simplex virus and cytomegalovirus. Their strong clinical track record, well-documented efficacy, and broad application in both acute and chronic viral infections position this class as a vital cornerstone of antiviral treatment.
Meanwhile, other segments like reverse transcriptase and protease inhibitors remain essential, especially in managing HIV and hepatitis, contributing to a diverse and evolving therapeutic landscape.
The branded drugs segment is expected to lead the global antiviral drugs market in 2025, commanding a 54.3% share. This dominance is attributed to factors such as widespread accessibility, expedited regulatory approvals, and strong physician and hospital endorsements. Branded antivirals typically benefit from substantial marketing efforts, patient awareness initiatives, and well-established distribution partnerships.
These advantages ensure rapid uptake in both hospital and outpatient settings. Although generic drugs offer cost-effective alternatives and are gaining popularity, branded antivirals continue to enjoy clinical preference, especially in critical care and first-line treatment scenarios where reliability and proven outcomes are prioritized.
Hospital pharmacies are anticipated to dominate the global antiviral drugs market in 2025, with a projected 36.1% share, due to their integral role in treating acute and life-threatening viral infections. These settings manage complex antiviral regimens, including intravenous treatments for hospitalized patients suffering from severe influenza, HIV complications, or hepatitis flare-ups.
Hospitals are often the first line of defence during outbreaks and pandemics, ensuring rapid and controlled access to antiviral drugs. Although retail and online pharmacies are expanding, particularly through telemedicine and home delivery models, the critical nature of hospital-based antiviral administration secures their lead in the overall distribution landscape.
To learn more about this report, Download Free Sample
North America is projected to lead the global antiviral drugs market in 2025, accounting for a 39.7% share. This dominance is fuelled by the region’s advanced healthcare infrastructure, strong presence of pharmaceutical giants, and high incidence of chronic viral infections such as HIV and hepatitis.
Significant investment in R&D, coupled with a supportive regulatory environment through agencies like the FDA, enables rapid development and approval of cutting-edge antiviral therapies. Additionally, broad insurance coverage and strategic public health programs enhance patient access to both branded and generic antivirals, ensuring continued market leadership.
Europe stands as a key contributor to the global antiviral drugs market, supported by increased public health initiatives and robust pharmaceutical innovation. Countries such as Germany, France, and the UK are actively investing in antiviral drug development and vaccination campaigns, particularly in response to emerging viral threats. The region’s strict regulatory standards and emphasis on healthcare access drive the adoption of high-quality antiviral therapies.
Furthermore, the rise of antimicrobial resistance and aging populations further accelerate demand for effective viral treatments, reinforcing Europe’s position as a mature and strategically important market.
The United States dominates the North American antiviral drugs market, driven by advanced healthcare infrastructure, strong R&D capabilities, and the presence of major pharmaceutical players like Gilead Sciences, Merck, and Pfizer. The U.S. maintains a high prevalence of chronic viral infections such as HIV and hepatitis, ensuring consistent demand for both branded and generic antiviral therapies. Government initiatives like the “Ending the HIV Epidemic” plan, along with robust insurance coverage, facilitate widespread access to antiviral medications and continued innovation in the field.
Canada contributes significantly to regional growth, benefiting from a publicly funded healthcare system and rising awareness of infectious disease prevention. The country's strong regulatory framework and participation in global vaccine and antiviral development programs support market expansion. Canada also promotes early adoption of new therapies through streamlined approval processes and national health campaigns, reinforcing North America’s leadership in antiviral drug innovation and access.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 67.04 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.3% | 2032 Value Projection: | USD 96.30 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
AbbVie Inc., GSK plc, Dr. Reddy's Laboratories Ltd., F. Hoffmann-La Roche Ltd, Bristol-Myers Squibb Company, Cipla, Aurobindo Pharma, Gilead Sciences, Inc., Merck & Co., Inc., Zydus Group, Atea Pharmaceuticals, Johnson & Johnson Services, Inc., Sun Pharmaceutical Industries Ltd., Pfizer Inc., Torrent Pharmaceuticals Ltd., Arbutus Biopharma, Divi's Laboratories Limited, RedHill Biopharma Ltd., and ViiV Healthcare Pty Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients