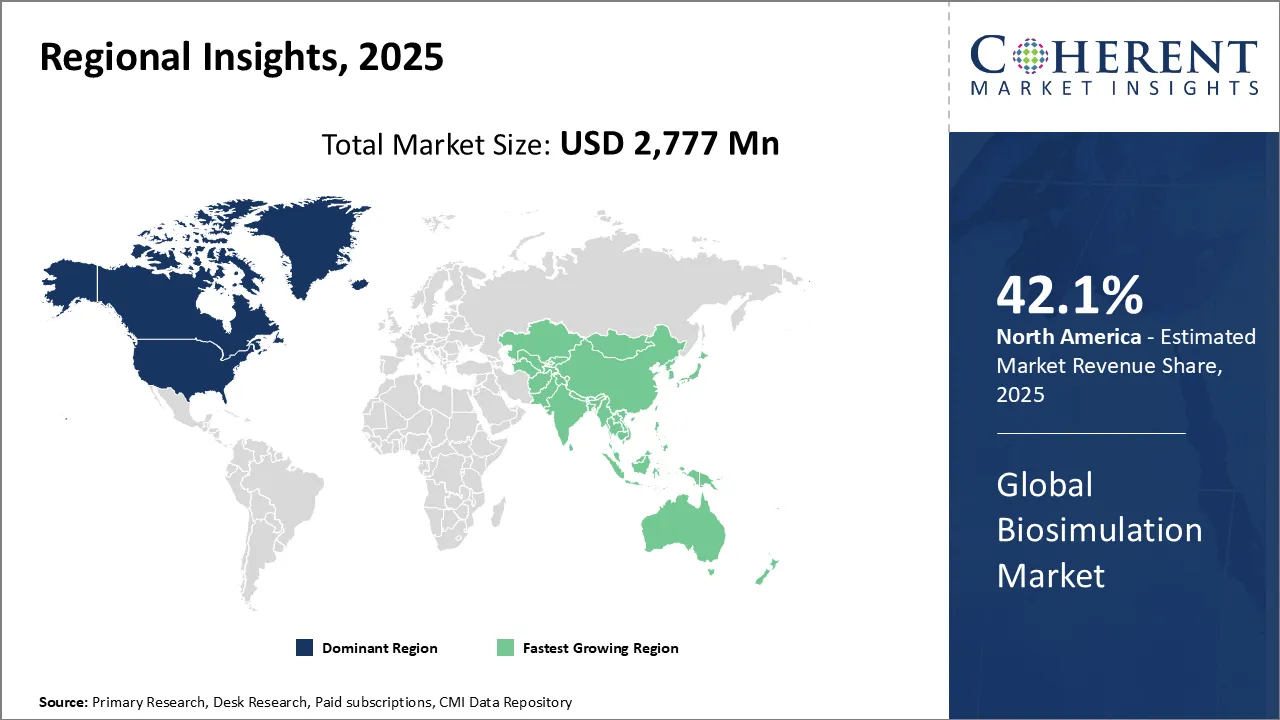
Global Biosimulation Market is expected to reach USD 8,689.9 Mn by 2032, from USD 2,777 Mn in 2025, exhibiting a CAGR of 17.7% during the forecast period.
Pharmaceutical and biotechnology companies are rapidly expanding the biosimulation market growth by adopting modeling and simulation tools to improve drug discovery and development. These tools allow researchers to test drug behavior virtually, enhancing accuracy while saving time and cost in research. The growing demand for personalized medicine, complex biologics, and regulatory encouragement for model-informed drug development are fueling this growth. Moreover, advancements in computing, artificial intelligence, and cloud technologies are making biosimulation more accessible and essential in modern healthcare innovation.
|
Current Events |
Description and its impact |
|
Geopolitical Developments |
|
|
Technological Advancements |
|
|
Regulatory and Policy Changes |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
AI plays a transformative role in biosimulation by enhancing the accuracy, speed, and predictive power of drug development models. Through machine learning and advanced analytics, AI can analyze vast biological and clinical datasets to identify patterns, optimize virtual trials, and simulate drug behavior in humans without relying heavily on animal testing. It enables the creation of more realistic digital twins, supports personalized medicine by predicting individual responses, and improves decision-making across discovery to regulatory stages. By integrating AI, biosimulation becomes more efficient, data-driven, and ethical, accelerating innovation while reducing costs and development risks in the pharmaceutical industry.
In April 2025, Certara, Inc. (Nasdaq: CERT), a global leader in model-informed drug development, introduced the Non-Animal Navigator™, a solution that helps biopharmaceutical companies align with the FDA’s Roadmap to Reducing Animal Testing in Preclinical Safety Studies. This initiative enables faster development, lower costs, and stronger predictive evidence, marking a shift toward more predictive, efficient, and ethical model-informed approaches. It reflects the growing industry adoption of scientifically robust, AI-enabled biosimulation and other new approach methodologies (NAMs) to enhance strategic decision-making and success across all stages of drug development.
Software holds the largest market share of 58.5% in 2025. Pharmaceutical and biotechnology companies are driving the growth of biosimulation software by adopting it to shorten drug development timelines and lower costs. They use these tools to simulate drug behavior and improve clinical outcomes. Advancements in artificial intelligence, high-performance computing, and cloud technologies are strengthening software performance. Moreover, regulators are increasingly supporting model-informed drug development, while the growing focus on personalized medicine is further boosting the adoption of biosimulation software across research institutions and healthcare organizations. For instance, in June 2024, Simulations Plus, Inc., a top provider of modeling and simulation software for pharmaceutical safety and efficacy, has acquired Pro-ficiency Holdings, Inc. and its subsidiaries, a leader in simulation-based solutions for clinical and commercial drug development.
Pharmaceutical companies are driving the expansion of biosimulation in drug development by using simulation tools to enhance efficiency, accuracy, and safety in creating new therapies. They apply these tools to predict pharmacokinetics, pharmacodynamics, and toxicity, which helps minimize trial failures and reduce development costs. Regulators are actively supporting model-informed approaches, while advances in artificial intelligence and computational technologies are boosting adoption. The rising demand for personalized medicine and biologics further strengthens the use of biosimulation for optimized, patient-specific drug development. For instance, Biosimulation company Cellworks launches a precision oncology drug development business, aiming to speed up promising therapies in pharma pipelines and revive previously studied, unapproved candidates. This is further accelerating the biosimulation market demand.
Hospital pharmacies are actively adopting biosimulation to optimize drug dosing, enhance patient safety, and improve therapeutic outcomes. Pharmacists use these simulation tools to monitor drug therapy, manage complex treatments, and personalize care, particularly for biologics and high-risk medications. Advances in digital technologies, data integration, and clinical decision-support systems allow hospital pharmacies to apply model-informed dosing efficiently. Additionally, increasing regulatory emphasis on evidence-based therapy and the growing focus on precision medicine are motivating hospitals to incorporate biosimulation into their pharmacy operations.
To learn more about this report, Download Free Sample
North America dominates the overall market with an estimated share of 42.1% in 2025. North America is actively transforming its biosimulation market through advancements in artificial intelligence, cloud computing, and digital twin technologies. Companies are integrating these innovations to make drug development more accurate and efficient. The FDA and other regulatory bodies are increasingly adopting model-informed drug development approaches, which accelerates industry uptake. Pharmaceutical firms, research institutions, and technology providers are collaborating to drive innovation and develop advanced biosimulation tools, firmly establishing North America as a leader in this rapidly evolving market.
The Asia Pacific region is rapidly advancing its biosimulation market through technological innovations and growing investments in biotechnology research and development. China, India, and Japan are leading efforts by expanding their pharmaceutical industries and strengthening regulatory frameworks to support biosimulation adoption. Pharmaceutical companies are leveraging the rise of personalized medicine and biologics to utilize simulation tools for more efficient drug development. Moreover, collaborations among research institutions, technology providers, and industry players are actively driving innovation and accelerating market growth across the region.
Advancements in artificial intelligence (AI), machine learning (ML), and computational biology are actively transforming the U.S. biosimulation market. Companies such as Certara and Simulations Plus are driving this change by integrating AI and ML into their biosimulation platforms to improve predictive accuracy and efficiency in drug development. The software segment dominates the market as pharmaceutical companies increasingly adopt AI-integrated tools for early-stage drug development, leveraging technological innovations to accelerate research and optimize outcomes.
Advancements in artificial intelligence (AI), computational biology, and supportive government policies are actively driving the growth of China’s biosimulation market. The country’s expanding pharmaceutical sector and rising investments in biotechnology research are further accelerating this trend. Large pharmaceutical companies in China are increasingly adopting biosimulation technologies to customize treatments based on genetic profiles, reflecting a strong focus on personalized medicine. This strategic push for precision medicine is optimizing drug development processes and establishing China as a major player in the global biosimulation market.
Artificial intelligence (AI) is revolutionizing drug discovery by enabling the development of programmable virtual humans—dynamic, multiscale models that simulate drug actions from molecular to phenotypic levels. These AI-driven models bridge the translational gap between early discovery and late development, offering a transformative path to optimize therapeutic efficacy and safety earlier than ever before. This advancement enhances the predictive accuracy of biosimulation tools, facilitating more efficient drug development processes.
The growing emphasis on personalized medicine is driving the adoption of biosimulation technologies to model patient-specific responses to therapies. Biosimulation tools enable the simulation of individual patient profiles, allowing for the optimization of drug dosages and treatment plans tailored to genetic and phenotypic variations. This approach enhances the efficacy and safety of treatments, aligning with the shift towards more individualized healthcare strategies.
Biosimulation is increasingly being applied in areas beyond traditional drug discovery, such as toxicology, pharmacokinetics, and disease modeling. These applications enable researchers to predict the effects of substances on biological systems, model disease progression, and optimize therapeutic interventions. The versatility of biosimulation tools enhances their value across various stages of biomedical research and development, supporting the advancement of personalized medicine and targeted therapies.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2,777 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 17.7% | 2032 Value Projection: | USD 8,689.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Certara, L.P., Simulations Plus, Inc., Dassault Systèmes (Accelrys), Genedata AG, LeadScope, Inc., Compugen Inc., Schrödinger, LLC, In Silico Biosciences, Inc., Advanced Chemistry Development, Inc., Chemical Computing Group ULC, Physiomics PLC, and Pharmaceutical Product Development, LLC (Evidera) |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients