Global contract pharmaceutical manufacturing market is estimated to be valued at USD 232.28 Bn in 2025 and is expected to reach USD 450.07 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.9% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The contract pharmaceutical manufacturing market is expected to witness significant growth over the forecast period. This growth is attributed to the increasing complexity of drug development process which is compelling pharmaceutical companies to opt for contract manufacturing. Moreover, the rising demand for generics and biologics is further the driving market growth. Additionally, growing focus of pharmaceutical companies on core competencies is resulting in the outsourcing of non-core activities like manufacturing to contract manufacturing organizations. However, high capital investment requirements for facilities and equipment as well as stringent regulatory guidelines can hamper the market growth. But focus on the modernization of manufacturing facilities coupled with emerging role of Artificial Intelligence (AI) and automation provides new avenues for contract pharmaceutical manufacturers.
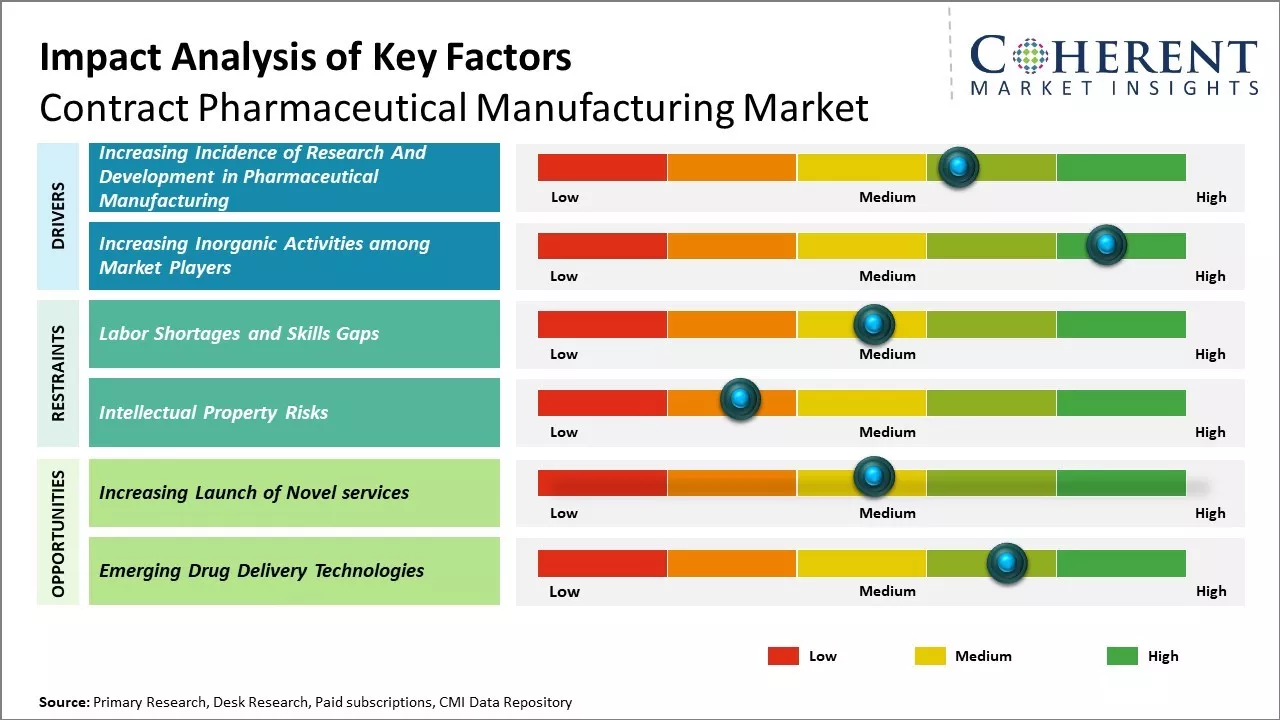
Increasing Incidence of Research and Development in Pharmaceutical Manufacturing
Demand for small molecules is considerably high compared to large molecules, owing to its various advantages in manufacturing and clinical trial studies. For instance, in November 2021, Pfizer Inc., a U.S.-based multinational pharmaceutical and biotechnology corporation, announced the successful acquisition of Trillium Therapeutics, a clinical stage immuno-oncology company developing innovative therapies for the treatment of cancer. According to the terms of the acquisition, Pfizer Inc. will purchase all shares of Trillium Therapeutics that are not already owned by Pfizer Inc. for a cash implied equity value of US$ 2.26 billion, or US$ 18.50 per share. This is 118% more expensive than Trillium Therapeutics’ 60-day weighted average price.
Get actionable strategies to beat competition: Download Free Sample
Increasing Inorganic Activities among Market PlayersIncreasing inorganic activities, such as agreements, partnerships, and collaborations, among market players are expected to drive the market growth over the forecast period. For instance, in September 2023, Parexel, a contract research organization (CRO), and Partex, a data-to-drugs pharma platform, inked a deal aiming to leverage artificial intelligence (AI)-powered solutions to accelerate drug discovery and development for biopharmaceutical customers worldwide and de-risk the assets in their portfolios.

To learn more about this report, Download Free Sample
Market Challenge – Labor Shortages and Skills GapsThe global contract pharmaceutical manufacturing market is facing significant challenges due to widespread labor shortages and skills gaps. A lack of qualified workers is restricting the production capacities of contract manufacturers and hindering their ability to meet rising customer demand. Several factors are exacerbating the talent crunch. The aging workforce in developed nations is depleting the pool of experienced professionals in pharmaceutical manufacturing. Younger generations often prefer less industrial careers, weakening recruitment pipelines. Stringent immigration policies during the pandemic also reduced the flows of skilled overseas workers to countries with major contract manufacturing industries.
Market Opportunity – Increasing Launch of Novel services
Increasing adoption of organic growth strategies such as launch of novel services by the key market players is expected to drive the market growth over the forecast period. For instance, in December 2021, IQVIA, a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry, launched new research nursing and phlebotomy services to offer access to a global network of high-quality, credentialed professionals for patients participating in clinical trials. The IQVIA research nursing and phlebotomy teams possess the capability to conduct a diverse range of assessments, collect data and specimens, and provide assistance in the direct delivery and administration of investigational products to patients.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
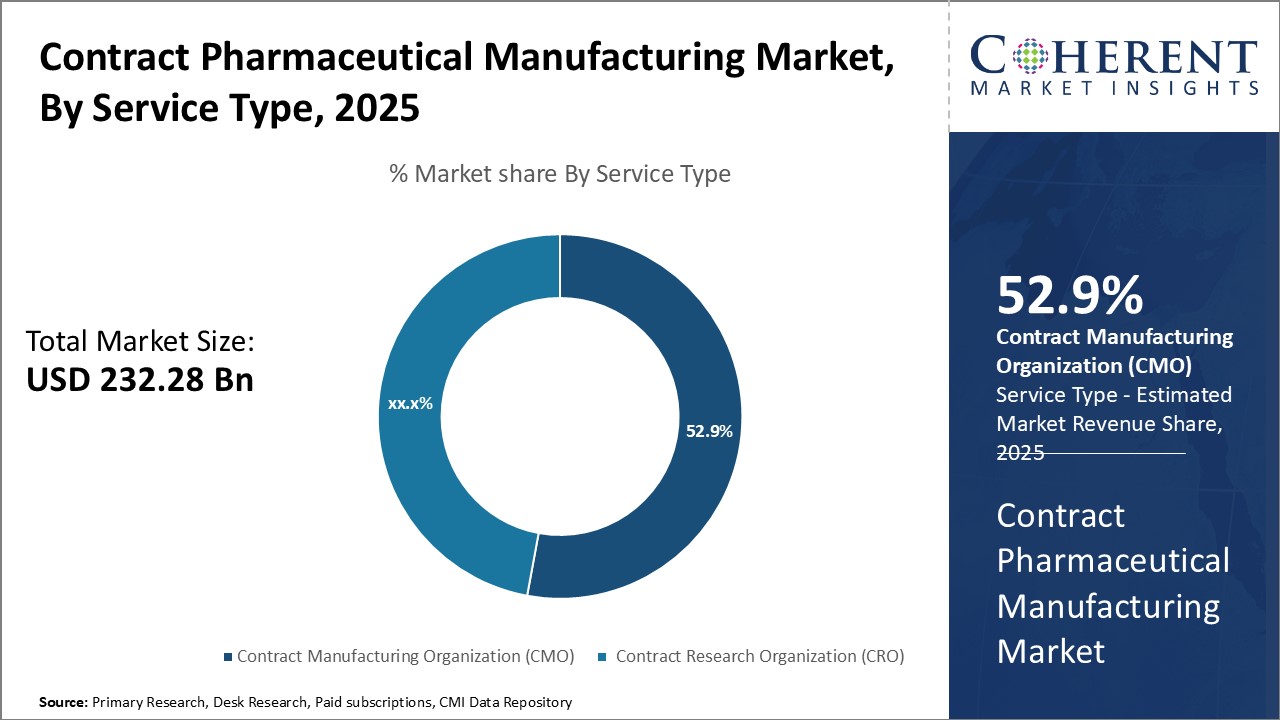
Insights, By Service Type: Contract Manufacturing Organization (CMO) contributes the highest share of the market owing to their end-to-end capabilitiesService type segment is sub-segmented into contract manufacturing organization (CMO) and contract research organization (CRO). The contract manufacturing organization (CMO) segment is estimated to hold 52.9% of the market share in 2025, owing to their robust end-to-end capabilities. CMOs provide extensive integrated services right from drug development and manufacturing to packaging and supply chain management. They have specialized expertise across various drug formulations like small molecules, biologics, injectables, etc. Their flexibility to scale up or down operations as per clients' changing needs is a major attraction. CMOs also offer strategic advantages to pharmaceutical companies like access to advanced manufacturing technologies and facilities without huge capital investment. This allows drug makers to focus on their core competencies of R&D and commercialization. The outsourcing of manufacturing activities to CMOs helps reduce fixed costs and ensures business continuity. Their global manufacturing footprint and experience in dealing with complex regulatory requirements of international markets provide pharmaceutical clients an easy entry into new geographies. Further, CMOs guarantee a high standard of quality and reliability through stringent compliance with CGMP norms.
Insights, By Molecule Type: Small molecules dominate the global contract pharmaceutical manufacturing market owing to their higher adoption across therapy areas
Molecule type segment is sub-segmented into small molecule and large molecule. The small molecule segment is estimated to hold 58.8% of the market share in 2025 due to the higher adoption of small molecule drugs across various therapeutic categories. A majority of existing drugs in the market are small molecule compounds which are relatively easier to develop and manufacture compared to large molecule biologics. Areas like cardiovascular, anti-inflammatory, anti-diabetic, anti-depressants, etc. extensively using small molecule-based medications. Their cost-effectiveness, well-established chemistry manufacturing and controls procedures, and simpler supply chain needs provide an advantage over large biologics. Further, the streamlined regulatory pathways and approvals for generic versions of blockbuster small molecule drugs boost their dominating presence. Compared to biologics, small molecules have a longer track record, well-known safety profiles, and mechanisms of action. This makes them a relatively lower-risk option for pharmaceutical companies. The difficulty and exorbitant costs associated with biosimilar development compared to generic versions of small molecules further consolidate their leading market share in contract manufacturing. Overall, the breadth of small molecule presence notably contributes to their ascendency in the global marketplace.
Need a Different Region or Segment? Download Free Sample
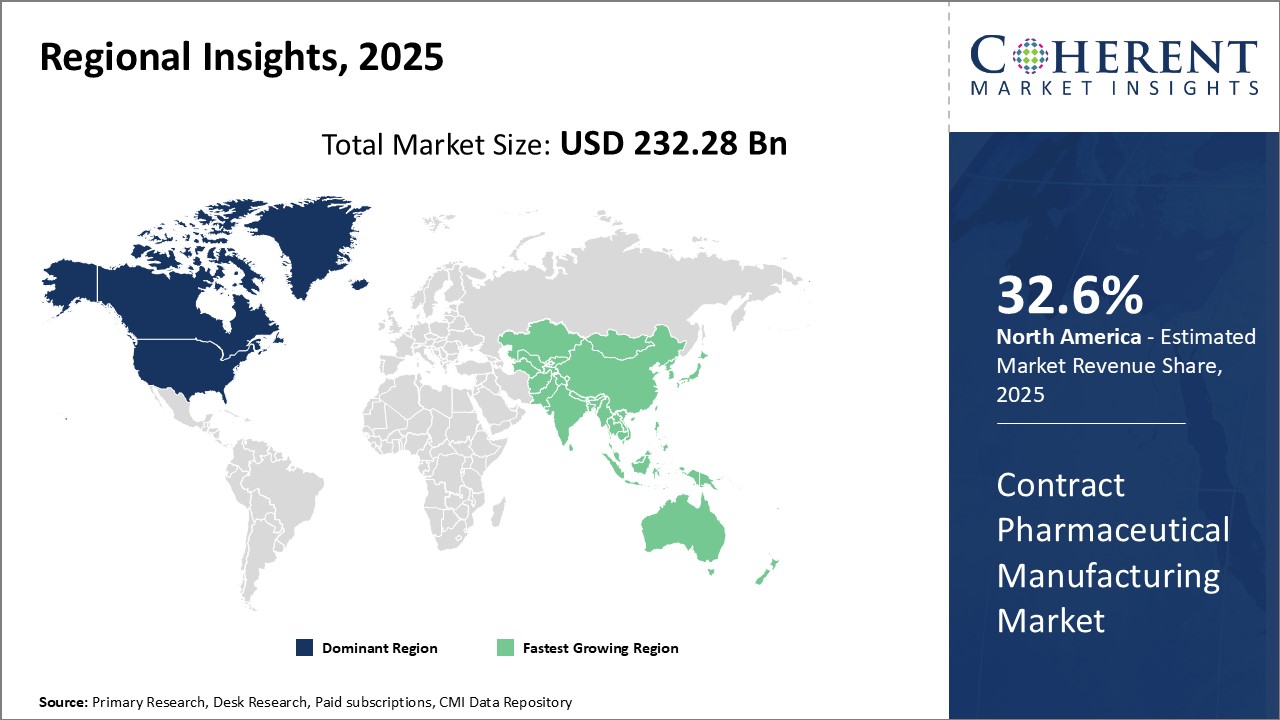
North America remains the dominant region in the global contract pharmaceutical manufacturing market and is estimated to hold 32.6% of the market share in 2025 with the U.S. being the major revenue generator. This can be attributed to the heavy presence of large pharmaceutical companies as well as contract development and manufacturing organizations in the region. Additionally, a large population opting for medicines manufactured under rigorous quality standards further aids the market growth. Despite rising manufacturing costs, North American companies prefer local manufacturing partnerships to achieve regulatory compliance and timely deliveries.
Asia Pacific has emerged as the fastest growing regional market for contract pharmaceutical manufacturing in recent years. Several factors have contributed towards this, foremost being proactive government support and initiatives to lure investments in pharmaceutical production. Countries like India and China offer low production costs, availability of skilled workforce as well as proximity to growing pharma markets of Asia Pacific and Africa. Additionally, these nations have built expertise across contract manufacturing services including active pharmaceutical ingredients production, generics development, and finished dosage formulation.
Contract Pharmaceutical Manufacturing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 232.28 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.9% | 2032 Value Projection: | USD 450.07 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
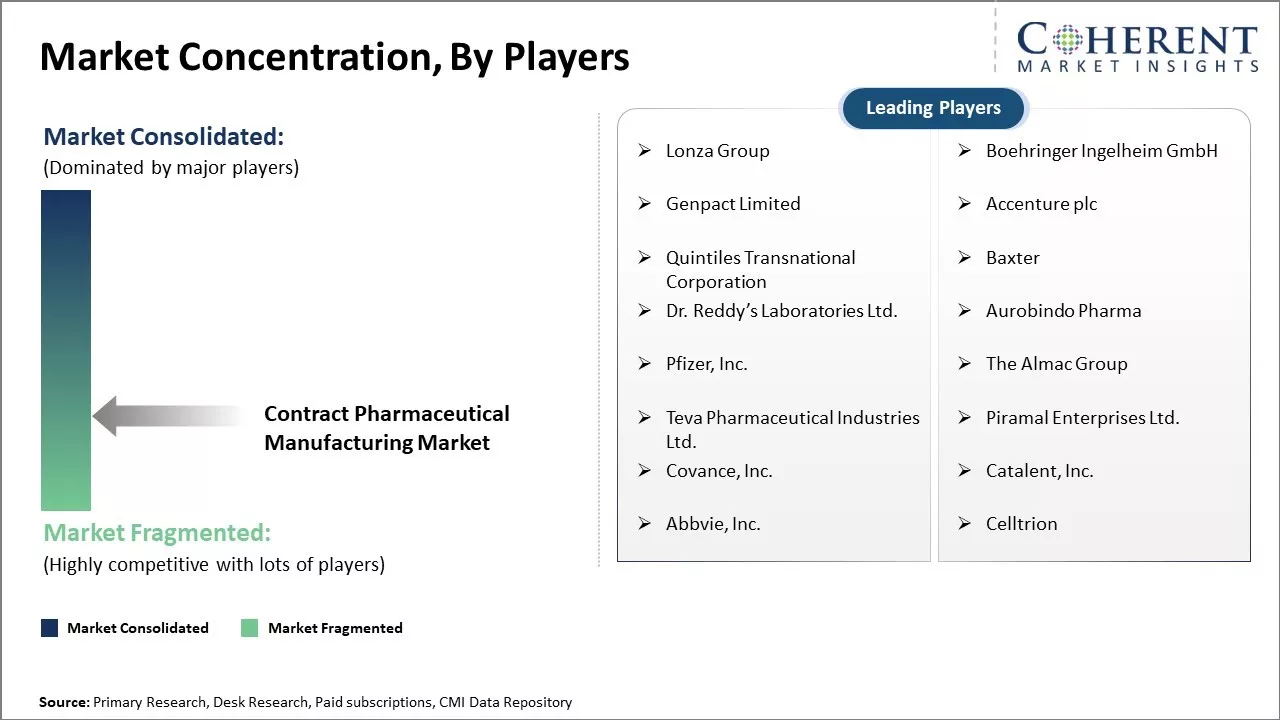
Lonza Group, Boehringer Ingelheim GmbH, Genpact Limited, Accenture plc, Quintiles Transnational Corporation, Baxter, Dr. Reddy’s Laboratories Ltd., Aurobindo Pharma, Pfizer, Inc., The Almac Group, Teva Pharmaceutical Industries Ltd., Piramal Enterprises Ltd., Covance, Inc., Catalent, Inc., Abbvie, Inc., and Celltrion |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Contract pharmaceutical manufacturing is engaged in conducting various kinds of drug production and research activities for different pharmaceutical companies. In the present time, there is an extensive need for pharmaceutical contract research and manufacturing, as drug manufacturers are facing increased research and manufacturing costs resulting due to the expiration of many older drugs patents, competition from drug industry, and stringent government regulations for new drug development. Pharmaceutical manufacturers are capable to reduce manufacturing and research & development costs by outsourcing several processes that were previously done in-house, ranging from initial drug research studies to the entire manufacturing process.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients