The coronavirus (COVID-19) outbreak was first reported on December 31, 2019, in Wuhan, China. The World Health Organization declared COVID-19 as a pandemic on March 11, 2020. COVID-19 infection is caused by the novel SARS-CoV-2, which is a member of single stranded RNA coronavirus family. Some of the common symptoms observed in COVID-19 patients include dry cough, fever, and fatigue while some less common symptoms include muscle pain, nausea, diarrhea, loss of smell, vomiting, chills, and conjunctivitis. Sometimes, severe symptoms such as shortness of breath, loss of appetite, and others may be observed in the COVID-19 patients. The COVID-19 rapid test kits are either rapid antigen test kits or rapid antibody test kits.
The global COVID-19 rapid diagnostic test market is estimated to be valued at US$ 3,846.9 Mn in 2021 and is expected to exhibit a CAGR of 8.8% during the forecast period (2021-2028).
Figure 1.Global COVID-19 Rapid Diagnostic Test Market Share (%) in Terms of Value, by Sample Type, 2021
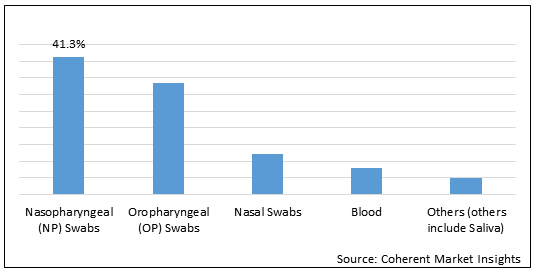
To learn more about this report, Download Free Sample
Increasing acquisition and collaboration among the key players is expected to drive the market growth during the forecast period
The increasing acquisition and collaboration among the key players is expected to drive the global COVID-19 rapid diagnostic test market growth during the forecast period. For instance, in February 2021, BD, a U.S.-based medical device company, collaborated with Scanwell Health, a California-based smartphone-enabled at-home medical tests manufacturer, to create an at-home lateral flow rapid antigen test for SARS-CoV-2 using a BD antigen test and the Scanwell Health mobile app.
COVID-19 Rapid Diagnostic Test Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2020 | Market Size in 2021: | US$ 3,846.9 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2021 to 2028 |
| Forecast Period 2021 to 2028 CAGR: | 8.8% | 2028 Value Projection: | US$ 6,952.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott Laboratories, F. Hoffmann-La Roche AG, Cardinal Health, Inc., Alfa Scientific Designs, Inc., Acon Laboratories, Inc., Thermo Fisher Scientific Inc., Danaher Corporation, PerkinElmer, Inc., Bio-Rad Laboratories, Inc., and Creative Diagnostics |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2.Global COVID-19 Rapid Diagnostic Test Market Share (%), by Test Type, 2021
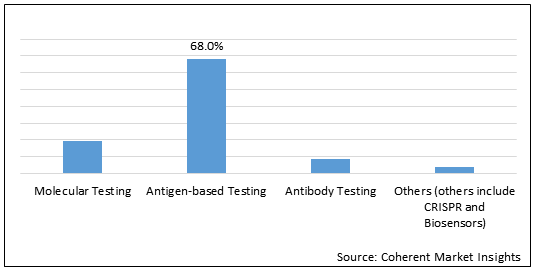
To learn more about this report, Download Free Sample
Increasing product launches and regulatory approvals is expected to drive the COVID-19 rapid diagnostic test market growth
Increasing product launches and regulatory approvals is expected to drive the COVID-19 rapid diagnostic test market growth during the forecast period. For instance, in September 2020, Laboratory Corporation of America Holdings, a U.S.-based testing laboratory company, launched a combined diagnostic test for flu, respiratory syncytial virus (RSV), and COVID-19.
Moreover, in September 2020, Mammoth Biosciences, a U.S.-based biotechnology company, received emergency use authorization from the U.S. FDA for its SARS-CoV-2 DETECTR Reagent Kit, which is based on CRISPR technology.
Global COVID-19 Rapid Diagnostic Test Market– Impact of Coronavirus (COVID-19) Pandemic
According to the Coronavirus (COVID-19) Weekly Epidemiological Update by the World Health Organization, over 245,403,909 cases and 4,981,395 deaths due to coronavirus (COVID-19) were reported till October 27, 2021, across the globe. During the COVID-19 pandemic, pharmaceutical business, and likewise biotechnology firms are facing difficulties due to the interruption in supply chains.
The global incidence of coronavirus is escalating demand for advanced diagnostic test kits, which is expected to boost growth of the COVID-19 rapid diagnostic test market.
Increasing demand of product launches for the diagnosis of COVID-19 is expected to drive the COVID-19 rapid diagnostic test market growth. For instance, in October 2020, Cipla Inc., an Indian pharmaceutical company, launched its new COVID-19 diagnostic test called ELIFast. ELIFast is based on ELISA (Enzyme linked immunosorbent assay), which detects the antibodies against SARS-CoV-2.
Global COVID-19 Rapid Diagnostic Test Market: Restraint
Product recalls, lack of skilled healthcare professionals, and less efficacy of rapid COVID-19 diagnostic tests as compared to laboratory setting are the factors that are expected to hinder growth of the global COVID-19 rapid diagnostic test market over the forecast period. For instance, in May 2020, India recalled rapid-antibody COVID-19 testing kits from two Chinese in-vitro diagnostics companies, namely Guangzhou Wondfo Biotech Co. and Zhuhai Livzon Diagnostics, Inc. due to low accuracy and inconsistent results delivered by the COVID-19 test kits.
Moreover, on October 1, 2021, Ellume Limited, an Australia-based digital diagnostics company, issued a recall of approximately 427,000 of its COVID-19 home tests. In total, approximately 42,000 affected tests were used and produced false positive results.
Key Players
Major players operating in the global COVID-19 rapid diagnostic test market include Abbott Laboratories, F. Hoffmann-La Roche AG, Cardinal Health, Inc., Alfa Scientific Designs, Inc., Acon Laboratories, Inc., Thermo Fisher Scientific Inc., Danaher Corporation, PerkinElmer, Inc., Bio-Rad Laboratories, Inc., and Creative Diagnostics.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients