Dextrose is also called as glucose, as they are chemically identical. It plays a vital role in providing energy to body cells. Dextrose injection is a non-pyrogenic and sterile solution for caloric supply and fluid replenishment by a single dose. Route of administration of dextrose injection is intravenous i.e. into the vein. Dosage of dextrose injection depends upon the weight, age, and clinical condition of the patient. Dextrose 5% in water is injected into the vein to replace lost fluids and it also provides carbohydrates to the body. Dextrose 5% in water is also used to treat hypoglycemia (low blood sugar), insulin shock, and dehydration (fluid loss). Dextrose is a carbohydrate, which is a part of nutrition. Dextrose solution along with amino acids and fats provide nutrition to the body. This is called as total parenteral nutrition. Dextrose solution (50%) is administered to people suffering from hyperkalemia, which is a condition in which potassium levels are high.
The global dextrose injection market is estimated to be valued at US$ 9,512.7 million in 2020 and is expected to exhibit a CAGR of 5.7% over the forecast period (2020-2027).
Figure 1. Global Dextrose Injection Market Share (%) in Terms of Value, By Region, 2020
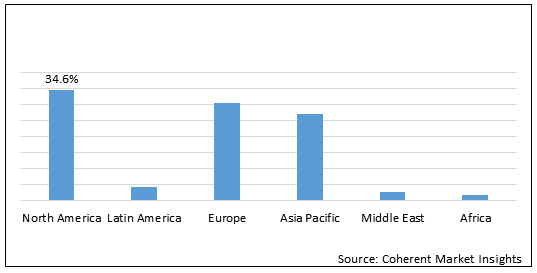
To learn more about this report, Download Free Sample
Increasing incidence of diabetes and conditions associated with diabetes such as hypoglycemia is expected to drive the market growth over the forecast period
According to the World Health Organization report published in June 2020, globally, the number of people with diabetes rose from 108 million in 1980 to 422 million in 2014.
Moreover, hypoglycemia is a condition in which blood sugar (glucose) level is lower than normal. Hypoglycemia is often related to diabetes treatment. According to a survey conducted by the Centers for Disease Control and Prevention (CDC), in 2016, more than 1.5 million people in the U.S. suffered from hypoglycemia.
Furthermore, various sports initiatives taken by the Indian government for the promotion of sports among individuals suffering from disabilities. ‘Khelo India’, National Sports Development Fund Scheme for promotion of sports among individuals suffering from disabilities. According to Khelo India Program, 1,000 students are selected annually and offered a sponsorship of 5 lakhs for 8 years. Such huge participation in sports is expected to boost growth of the global dextrose injection market. Athletes use dextrose injection to treat a variety of ligament, muscle, tendon, and joint pain.
Figure 2. Global Dextrose Injection Market Share (%), by Product Type, 2020
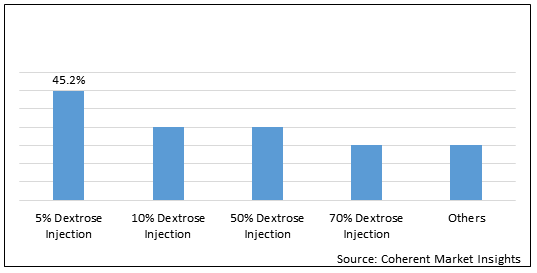
To learn more about this report, Download Free Sample
Increasing launch and approval of products is expected to drive the market growth over the forecast period
Key players operating in the market are focusing on novel product launches and approvals, in order to expand their product portfolio and increase presence in the global market.
For instance, in July 2020, Hikma Pharmaceuticals PLC (Hikma), a multinational pharmaceutical company, launched Dacarbazine for Injection with dextrose solution for the treatment of metastatic malignant melanoma. The reconstituted solution may be further diluted with 5% dextrose injection or sodium chloride injection, and administered as an intravenous infusion.
In April 2018, SteriMAx Inc., company engaged in the production, development, marketing, and distribution of essential hospital and retail pharmacy products, launched a new drug Bupivacaine Hydrochloride in 8.25% dextrose injection indicated for spinal anesthesia. It is a subarachnoid injection (spinal block).
For instance, in 2017, Clindamycin in dextrose injection (D5W) made by Water Street Healthcare Partners, a private equity firm, which focuses extensively on building market leading companies in healthcare and Celerity Pharmaceuticals LLC, which provides drug development services, was approved by the U.S. Food and Drug Administration. Clindamycin in dextrose injection (D5W) is used to treat bacterial infections such as pneumonia by inhibiting growth of bacteria in the body.
Dextrose Injection Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2019 | Market Size in 2020: | US$ 9,512.7 Mn |
| Historical Data for: | 2016 to 2019 | Forecast Period: | 2020 to 2027 |
| Forecast Period 2020 to 2027 CAGR: | 5.7% | 2027 Value Projection: | US$ 14,056.6 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer Inc., Amphastar Pharmaceuticals Inc., Shangai Haixin group Co. Ltd., Seqirus GmbH, Baxter International Inc., Shandong Qidu Pharmaceutical, Sanctus drugs and pharmaceuticals Pvt., Hospira Inc., B Braun Medical Ltd., Aurobindo Pharmaceuticals Ltd., and SteriMAx Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Dextrose Injection Market – Impact of Coronavirus (Covid-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization declared it a public health emergency. According to the World Health Organization’s report, the manifestation of coronavirus disease (COVID-19) resulted in more than 31 million infected individuals worldwide as of September 21, 2020. Furthermore, key players operating in the market are focusing on novel product launch in order to combat COVID -19. For instance, in May 2020, Hikma Pharmaceuticals PLC (Hikma, Group), a multinational pharmaceutical company, announced the launch of Propofol Injectable Emulsion, USP, 20 mL, 50 mL, and 100 mL vials with 5% dextrose injection, in the U.S. through its US affiliate, Hikma Pharmaceuticals USA Inc. Propofol injectable emulsion is provided as a ready-to-use formulation. However, should dilution be necessary, it should only be diluted with 5% Dextrose Injection, USP and it should not be diluted to a concentration less than 2 mg/mL because it is an emulsion.
Propofol Injectable Emulsion is indicated for the maintenance of sedation and anesthesia for intubated, mechanically ventilated adults in the intensive care unit. It is currently on the FDA's Drug Shortage List, following a surge in demand due to the increase in hospitalized, ventilated patients due to the COVID-19 pandemic.
Global Dextrose Injection Market - Restraint
Side effects caused by dextrose injection such as fever, infection at the site of injection, and blood clot at the site of injection, and voluntary product recall are major factors restraining growth of the dextrose injection market.
For instance, in February 2018, the U.S. Food and Drug Administration announced the recall of Hospira, Inc., a Pfizer company’s, one lot of 25% Dextrose Injection, USP, (Infant) pre-filled syringe to the hospital/user level due to the presence of particulate matter, identified as human hair, found within an internal sample syringe.
Key Players
Major players operating in the global dextrose injection market include Pfizer Inc., Amphastar Pharmaceuticals Inc., Shangai Haixin group Co. Ltd., Seqirus GmbH, Baxter International Inc., Shandong Qidu Pharmaceutical, Sanctus drugs and pharmaceuticals Pvt., Hospira Inc., B Braun Medical Ltd., Aurobindo Pharmaceuticals Ltd., and SteriMAx Inc.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients