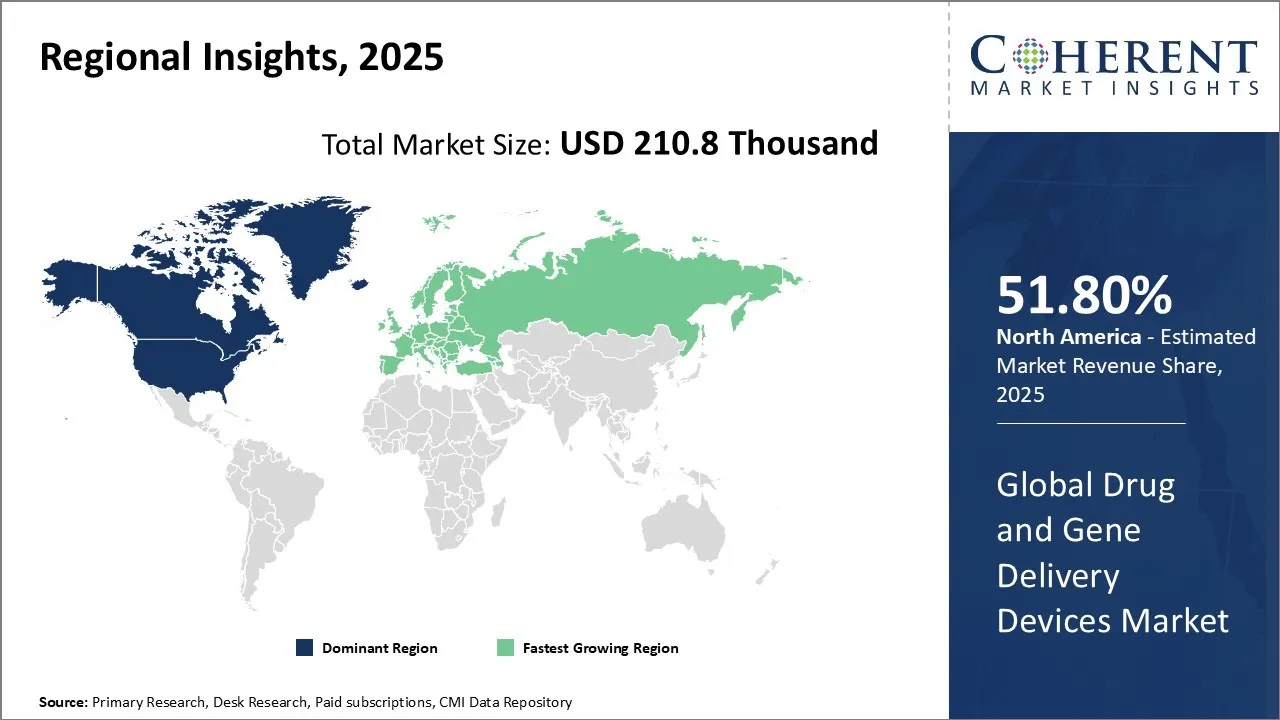
Drug and Gene Delivery Devices Market is estimated to be valued at USD 210.8 Th in 2025 and is expected to reach USD 454.5 Th in 2032, exhibiting a compound annual growth rate (CAGR) of11.6% from 2025 to 2032.
The global drug and gene delivery devices market is experiencing strong growth due to the rise in burden of chronic disease and high demand for drug and gene delivery devices. Moreover, increasing funding for cell and gene therapies and rise in approvals for gene therapies is expected to boost growth of the market. However, factors such as stringent rules and regulations and high risk of needlestick injuries are expected to hamper the market growth.
|
Current Events |
Description and its Impact |
|
Market Consolidation & Strategic Alliances |
|
|
Healthcare Cost Inflation Drivers |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The integration of artificial intelligence into drug and gene delivery devices has the potential to transform the delivery process by streamlining therapy and reducing side effects. AI algorithms facilitate researchers in predicting drug release profiles and offer dosage regimens to get efficient therapies. Ai-powered devices monitor patient for medication and provide real-time feedback, reliability, and improve safety.
In September 2024, Asimov launched the AAV Edge System, an integrated platform combining AI models, optimized host cells, and genetic tools for adeno-associated viral (AAV) gene therapy. This system aims to streamline the development process by addressing challenges related to safety, efficacy, manufacturability, and cost in gene therapy development.
In terms of product, the extension tube segment has emerged as the dominant force in the global drug and gene delivery devices market, capturing the largest share of 49.1%in 2025. The utilization of extension tube offers the delivery of medication to the patient by connecting to the other devices such as syringe pump. The extension tube is connected to the medical devices that uses standard luer lock or luer slip connector to facilitate the deliverance of infused substances into the patient.
In June 2024, Medtronic launched its Steerant™ Aortic Guidewire, a device that facilitates catheter placement and exchange during diagnostics and interventional procedures in the aorta, including endovascular aneurysm repair (EVAR) and thoracic endovascular aortic repair (TEVAR). This medical device features a super stiff design with single- and double-curve tip configuration.
Sub-retinal Injection Cannula Segment is expected to be the fastest growing segment in the drug and gene delivery devices market over the forecast period. The growth is attributed to the increasing demand for subretinal injection cannulas. Subretinal injection cannula is a more efficient and precise method of optical drug delivery for cell and gene therapies. This rigid device penetrates the retina, eliminating the need to create a separate retinotomy. They can also be used for low volume fluid drainage, such as draining subretinal PFCL. The luer lock hub allows a quick secure connection to extension tube.
In terms of commercialized drugs, Luxturna (Drug Delivery Devices) Segment is expected to dominate the market over the forecast period and this is attributed to the rise in approvals for gene therapies. Luxturna is the first directly administered gene therapy approved in the U.S. that targets a disease caused by mutations in a specific gene. Luxturna is highly demanding in drug delivery derived due to its unique gee therapy approach for treating inherited retinal disease such as Leber’s congenital amaurosis (LCA). Luxtura is proven to show improvement is patient’s vision and ability to move around obstacles, especially in dim light. The efficacy of the gene therapy was assumed to be 93%, the demonstrated success rate of Luxturna in Phase III trials.
In July 2024, the UPMC Vision Institute treated its first patient with Luxturna® (voretigene neparvovec-rzyl), marking a significant advancement in the clinical application of gene therapy for inherited retinal dystrophies (IRDs) caused by biallelic mutations in the RPE65 gene. The deliver has received approval by the U.S. FDA. It is designed to slow or halt retinal degeneration by delivering a functional copy of the RPE65 gene into the subretinal space.
To learn more about this report, Download Free Sample
Among regions, North America is expected to gain highest share of 51.8% in the market in 2025 due to the increasing prevalence of chronic diseases, such as cancer, development of gene therapies, increasing demand for drug and gene delivery devices, and introduction of novel devices in the region. For instance, according to the American Cancer Society (ACS), in 2025, more than 1,958,310 new cancer cases and 609,820 cancer deaths are projected to occur in the U.S. In 2024, the North America, it is estimated 2,041,910 new cancer cases are expected in, equating approximately 5,600 diagnoses daily. Ther are roughly round 618,120 cancer related deaths were projected to occur in the U.S. This in turn is driving the drug and gene delivery market growth.
Europe is expected to witness robust growth in the global drug and gene delivery devices market owing to the rise in burden of chronic diseases, such as infectious disease, cardiovascular disease, cancer, and diabetes, increasing demand for drug or gene delivery devices, and introduction of novel devices in these regions. For instance, according to the European Heart Network, each year, cardiovascular diseases (CVDs) cause more than 3.9 million deaths in Europe and around 1.8 million deaths in the European Union (EU). CVDs account for around 45% of all deaths in Europe and 37% of all deaths in the EU. This is further contributing to the drug and gene delivery devices market share.
The Aisa pacific region is expected to exhibit the fastest growing market share during the forecast period. The market in Asia pacific region is driven by rising prevalence of chronic and infectious disease. Along with that there is a rapidly growing biopharmaceutical industry and increasing urbanization which contributes to the growth of the market. According to WHO (World Health Organization) Aisa region estimates 2.37 million new cancer cases and 1.53 million cancer deaths in 2022. Vietnam had 442 pharmaceutical companies, the most in Southeast Asia, followed by Thailand with 395.
The U.S. drug and gene delivery devices market driven by advanced healthcare infrastructure, strong biopharma R&D ecosystem and high adoption of innovative delivery technology. the unites states has an increasing use of wearable drug delivery devices, gene therapies for rare disease and personalized medicine. The U.S. global pharmaceutic industry accounts for around 30-40% f the total market. This figure rises to approximately 45% of global pharmaceutical sales and 22% of global production.
China’s market for drug and gene delivery devices market is expecting a rapid growth, fueled by the country’s expanding healthcare access, government incentives for biotech ad medical device sectors, and increasing chronic disease prevalence. Moreover, the market in China is driven by the strong demand for injectable delivery devices and gene therapy platform, and localized manufacturing. Under innovative drug approval in China, for instance, from January to August 2024, China's National Medical Products Administration approved 31 innovative drugs, a rise of nearly 20% from the same period in the previous year, reflecting the country's commitment to supporting first-in-class and innovative drugs.
Self-administered drugs are medicines a person can take at home, without assistance. The growing trend towards the self-administration of drugs has increased the demand for convenient and easy-to-use drug delivery systems. Moreover, with the development of novel drug delivery technologies and the continued focus on patient-centric healthcare, the demand for novel drug delivery devices is also increasing with a rapid pace, worldwide. This trend is expected to continue over the forecast period, driving the drug and gene delivery devices market growth.
With the increasing prevalence of chronic/targeted diseases across the globe, the demand for safe and effective drug and gene delivery devices as well as demand for novel cell and gene therapies is also increasing with a rapid pace. Thus, players in the market are focusing on developing and/or launching novel devices or therapies to meet growing demand. This trend is expected to continue over the forecast period, driving the growth of the market.
One of the key factors expected to augment the growth of the global drug and gene delivery devices market during the forecast period is the increasing prevalence of chronic disease around the world. For instance, with the rise in burden of chronic diseases, such as infectious disease, cardiovascular disease, cancer, and diabetes, the demand for drug/gene delivery devices is also increasing with a rapid pace. According to the World Health Organization (WHO), cardiovascular diseases account for most NCD (noncommunicable disease) deaths, or 17.9 million people every year, followed by cancers (9.3 million), chronic respiratory diseases (4.1 million), and diabetes (2.0 million).
Another factor which is driving the growth of the global drug and gene delivery devices market is the increasing demand for gene and drug delivery devices around the world. For instance, a drug and gene delivery device is a medical device that enables a drug and gene to reach its site of action without reaching non-target cells, organs, and tissues. In May 2022, Jabil Healthcare launched the Qfinity autoinjector platform, a simple, reusable, and modular solution for subcutaneous (SC) drug self-administration, at a lower cost than market alternatives, supporting the emerging prioritization of sustainable drug delivery within the pharmaceutical industry.
Another factor which is hampering the growth of the global drug and gene delivery devices market is the high risk of needlestick injuries. Needlestick injuries (NSIs) are wounds caused by needles that accidentally puncture the skin. Needlestick injuries are one of the most important occupational hazards among healthcare workers (HCWs) worldwide. Needlestick injuries can lead to serious or fatal infections with blood-borne pathogens, such as hepatitis B virus, hepatitis C virus, and HIV. Thus, several regulatory authorities across the world are mandating the usage of safety syringes to help reduce the risk associated with needlestick injuries. It is estimated that 44.5% of healthcare workers at least one needlestick injuries (NSIs) occurrence annually. Every year, 385,000 healthcare workers in the United States and more than 1 million healthcare workers in Europe experience a percutaneous injury each year as a result of accidents with needles or sharps.
Increasing funding for cell and gene therapies is expected to offer significant growth opportunities for players in the drug and gene delivery devices market. For instance, in May 2023, ElevateBio has raised USD 401 million in a Series D financing round for advancing its technology platforms to expedite the design, production, and development of cell & gene therapies. Moreover, in may 2025, The Maryland Stem Cell Research Commission is allocating in excess of $18 million in funding through the Maryland Stem Cell Research Fund (MSCRF) to provide support for 52 researchers affiliated with various academic institutions and private enterprises within the state. These awards are designed to address a diverse array of therapeutic areas, which encompass hematologic disorders such as sickle cell disease, metabolic conditions including diabetes, various types of cancer, chronic pain, as well as diseases impacting the cardiovascular, musculoskeletal, digestive, and neurological systems.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 210.8 Th |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.6% | 2032 Value Projection: | USD 454.5 Th |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer, Inc., Novartis AG, Kite Pharma, Inc., Bluebird bio, Inc., Becton Dickinson and Company, Amgen, Inc., Vericel Corporation, uniQure N.V., Spark Therapeutics, Inc., Renova Therapeutics, Orchard Therapeutics plc, Kolon Tissue Gene, Inc., Human Stem Cell Institute, Dendreon Pharmaceuticals, Helixmith Co., Ltd (ViroMed Co., Ltd), Bausch & Lomb Incorporated, and Castle Creek Biosciences, Inc (Fibrocell Technologies, Inc.), among others. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: The field of drug and gene delivery involves development and delivery of drugs, genes, and gene products that ultimately alter protein expression and function of the cells, tissues, and living organisms.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients