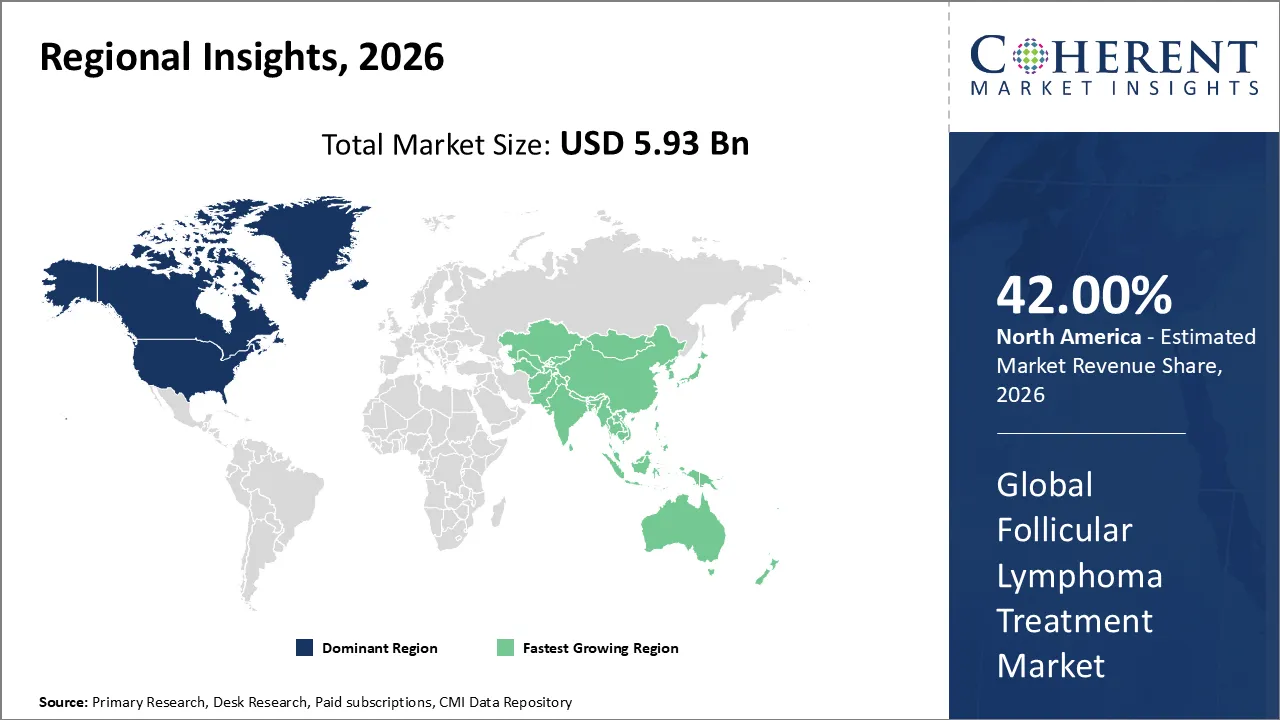
Follicular lymphoma treatment market is estimated to be valued at USD 5.93 Bn in 2026 and is expected to reach USD 9.41 Bn in 2033, exhibiting a compound annual growth rate (CAGR) of 6.8% from 2026 to 2033.
Follicular lymphoma can be treated on the basis of the acuteness of associated symptoms and on cancer growth rate. Radiation and chemotherapy are presently the responsive treatment for follicular lymphoma. However, radiation alone can be used for the treatment of follicular lymphoma in most of the cases. In more advanced cases chemotherapy or a monoclonal antibody rituximab, alone or the combination of both are used for the treatment of follicular lymphoma. Moreover, monoclonal antibodies are more predominant over the chemotherapy, as they act directly by targeting on tumor cells and thereby, increases immune cells that destructs tumor, which increases response to the treatment.
|
Current Event |
Description and its Impact |
|
FDA and EMA Regulatory Advances in CAR-T Cell Therapies |
|
|
Breakthrough CAR-T and Bispecific Antibody Clinical Trial Results |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
|
Region |
Average Pricing Range |
Notes on Accessibility |
|
North America (U.S., Canada) |
USD 350,000–450,000 for CAR‑T and bispecific antibodies; chemotherapy regimens ~USD 50,000–80,000 |
Insurance coverage helps, but high out‑of‑pocket costs remain for uninsured patients |
|
Europe (UK, Germany, France) |
USD 250,000–350,000 for advanced biologics; chemotherapy ~USD 40,000–70,000 |
Government reimbursement programs reduce patient burden; access varies by country |
|
Asia‑Pacific (China, Japan, India) |
India: USD 25,000–40,000 for chemotherapy/targeted therapy; Japan/China: USD 150,000–250,000 for biologics |
India offers lower‑cost care, while Japan and China adopt biologics with partial reimbursement |
|
Latin America (Brazil, Argentina) |
USD 100,000–200,000 for biologics; chemotherapy ~USD 30,000–50,000 |
Public healthcare systems slow adoption of advanced therapies |
|
Middle East & Africa |
USD 150,000–250,000 for biologics; chemotherapy ~USD 20,000–40,000 |
Limited availability; patients often travel abroad for CAR‑T or advanced biologics |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of treatment type, the targeted therapy segment is expected to hold 67.4% share of the market in 2026. Its dominance stems from precision medicines like monoclonal antibodies and PI3K inhibitors that selectively attack cancer cells while sparing healthy tissue. Reduced toxicity, improved survival rates, and integration into first-line regimens drive widespread adoption globally.
For instance, in November 2025, The FDA approved epcoritamab in combination with rituximab and lenalidomide for adults with relapsed follicular lymphoma. This targeted immunotherapy marks a major advance, reducing disease progression risk and improving response rates. Its precision, reduced toxicity, and chemotherapy‑free approach reinforce targeted therapy’s dominance in the follicular lymphoma treatment landscape.
In terms of end user, the hospitals segment is expected to lead the market with 48% share in 2026, fueled by advanced infrastructure, access to biologics, and ability to deliver complex therapies such as targeted regimens and stem cell transplants. Centralized patient care, clinical expertise, and integration with research trials reinforce hospitals as the primary treatment hubs.
For instance, in December 2025, A new MSK trial shows a three‑drug combination benefits people with relapsed follicular lymphoma. Conducted in hospital settings, it highlights advanced infrastructure, clinical expertise, and research integration. Hospitals remain central hubs for delivering complex therapies, reinforcing their leadership in follicular lymphoma treatment and patient care worldwide.
To learn more about this report, Download Free Sample
North America is expected to lead the follicular lymphoma treatment market with 42% share in 2026, due to high disease prevalence, advanced hospital infrastructure, and rapid adoption of targeted therapies. FDA approvals of innovative biologics, integration of clinical trials, and strong pharmaceutical presence reinforce the region’s dominance, ensuring widespread patient access and improved outcomes.
For instance, in December 2025, the FDA approved Roche’s Lunsumio VELO (mosunetuzumab) subcutaneous injection for relapsed or refractory follicular lymphoma in adults. This North America milestone reduces administration time to one minute, improves patient convenience, and strengthens targeted immunotherapy’s role in hospitals, reinforcing the region’s dominance in lymphoma treatment.
Asia Pacific is anticipated to be the fastest growing region, due to increasing cancer prevalence, expanding healthcare infrastructure, and greater access to advanced biologics. Government investments, growing clinical trial activity, and heightened awareness drive adoption of targeted therapies, making the region the fastest‑growing market segment globally.
For instance, in March 2025, Japan approved Chugai’s LUNSUMIO (mosunetuzumab) for relapsed or refractory follicular lymphoma after two prior therapies. This CD20/CD3 bispecific antibody offers targeted immunotherapy, enabling durable remission. Listed under national insurance, it expands patient access and reinforces Asia‑Pacific’s rapid growth in advanced lymphoma treatment.
In 2026, the U.S. drives demand for follicular lymphoma treatment due to high patient prevalence, rapid FDA approvals of targeted immunotherapies, and strong hospital infrastructure. Robust clinical trial activity, advanced biologics adoption, and pharmaceutical innovation reinforce the nation’s dominance, ensuring improved outcomes and expanding access to cutting‑edge therapies.
For instance, in November 2025, the FDA advanced North America’s leadership in follicular lymphoma treatment by approving Genmab’s Epkiniy (epcoritamab‑bysp) with rituximab and lenalidomide. This targeted immunotherapy combination offers a chemotherapy‑free option, improves patient outcomes, and underscores hospitals’ role in delivering complex biologics, reinforcing regional dominance in cancer care.
In 2026, China’s demand for follicular lymphoma treatment rises due to increasing cancer prevalence, NMPA approvals of targeted therapies like Tazverik, and expanding hospital infrastructure. Government healthcare investments, broader insurance coverage, and growing clinical trial activity enhance patient access, driving rapid adoption of advanced biologics and fueling market growth.
For instance, in March 2025, China’s NMPA approved Hutchmed’s Tazverik (tazemetostat) for relapsed or refractory follicular lymphoma after two prior therapies. As an EZH2 inhibitor, it offers a targeted option for patients with mutations or limited alternatives, expanding access and reinforcing Asia‑Pacific’s rapid growth in advanced lymphoma treatment.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 5.93 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 6.8% | 20323 Value Projection: | USD 9.41 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Roche, Spectrum Pharmaceuticals, Johnson and Johnson, CTI Biopharma, Celgene, AbbVie Inc., Novartis, Amgen, Merck & Co. (MRK), Seattle Genetics, Pharmacyclics/ Bristol-Myers Squibb (BMY), and Bayer AG’S |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The rising incidence of follicular lymphoma globally is a major growth driver. With more patients requiring advanced therapies, pharmaceutical companies and hospitals are expanding treatment options. This trend directly boosts the Follicular Lymphoma Treatment Market share, as innovative biologics and targeted therapies replace traditional chemotherapy, ensuring better outcomes and wider adoption across regions. According to Blood Cancer United, in the United States, FL takes place about 15,000 times a year, and the patients having an average age of approximately 60. The five-year survival rate is 87.7%, and younger patients have a better chance of surviving.
Governments worldwide are investing heavily in oncology infrastructure, insurance coverage, and clinical trials. These initiatives improve patient access to advanced therapies, particularly in Asia‑Pacific and emerging markets. Such support significantly raises Follicular Lymphoma Treatment Market demand, as more patients can afford and receive cutting‑edge biologics, driving rapid growth and regional expansion.
Collaborations between pharmaceutical companies and hospitals accelerate clinical trial activity, ensuring faster approvals and wider patient access to advanced therapies. Hospitals provide infrastructure and patient pools, while pharma firms contribute research and funding. Together, they enhance treatment efficacy, expand therapeutic options, and strengthen penetration, significantly influencing the Follicular Lymphoma Treatment Market forecast with sustained growth through 2026 and beyond.
For instance, in December 2025, MSKCC reported a three‑drug immunotherapy combination of epcoritamab‑bysp, rituximab, and lenalidomide, resulting in benefiting relapsed follicular lymphoma patients. Recently FDA‑approved, this trial highlights collaboration between hospitals and pharma, accelerating innovation and patient access. Such partnerships strengthen clinical pipelines and shape the Follicular Lymphoma Treatment Market forecast with rising demand.
The follicular lymphoma treatment market is expanding in response to the growing diagnosed patient population and advances in therapeutic options. Follicular lymphoma is a type of non-Hodgkin lymphoma that is slow-growing and makes up a large part of all NHL cases. This means that there is a constant need for effective ways to manage it. Recently, about 35,000 new cases were found in major markets, and the number of cases is growing, especially among older adults.
Monoclonal antibodies, chemotherapy, targeted small molecule inhibitors, and new cellular therapies are all examples of therapeutic modalities. Rituximab and other next-generation anti-CD20 monoclonal antibodies are important parts of first-line regimens. Anti-CD20 monoclonal antibodies such as rituximab and next-generation agents are key components of first-line regimens, with documented high response rates when used in combination with chemotherapy. Bispecific antibodies and CAR-T cell therapies are gaining traction, especially in relapsed or refractory populations, demonstrating meaningful objective response metrics in clinical settings.
Regional patterns show that North America is still the biggest market, due to a strong diagnostic infrastructure and a quick uptake of new treatments. More people in Europe and Asia-Pacific are getting treatment because there are more oncology care facilities and clinical research going on. Combining precision medicine with personalized treatment planning is changing the way we treat diseases by allowing us to create treatment plans that are specific to each patient and their disease.
Cost and accessibility are still important factors in adoption, and advanced therapies often need special delivery and monitoring. Still, ongoing clinical progress and new therapies in the pipeline are likely to give patients more treatment options and help them manage their diseases better over time.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients