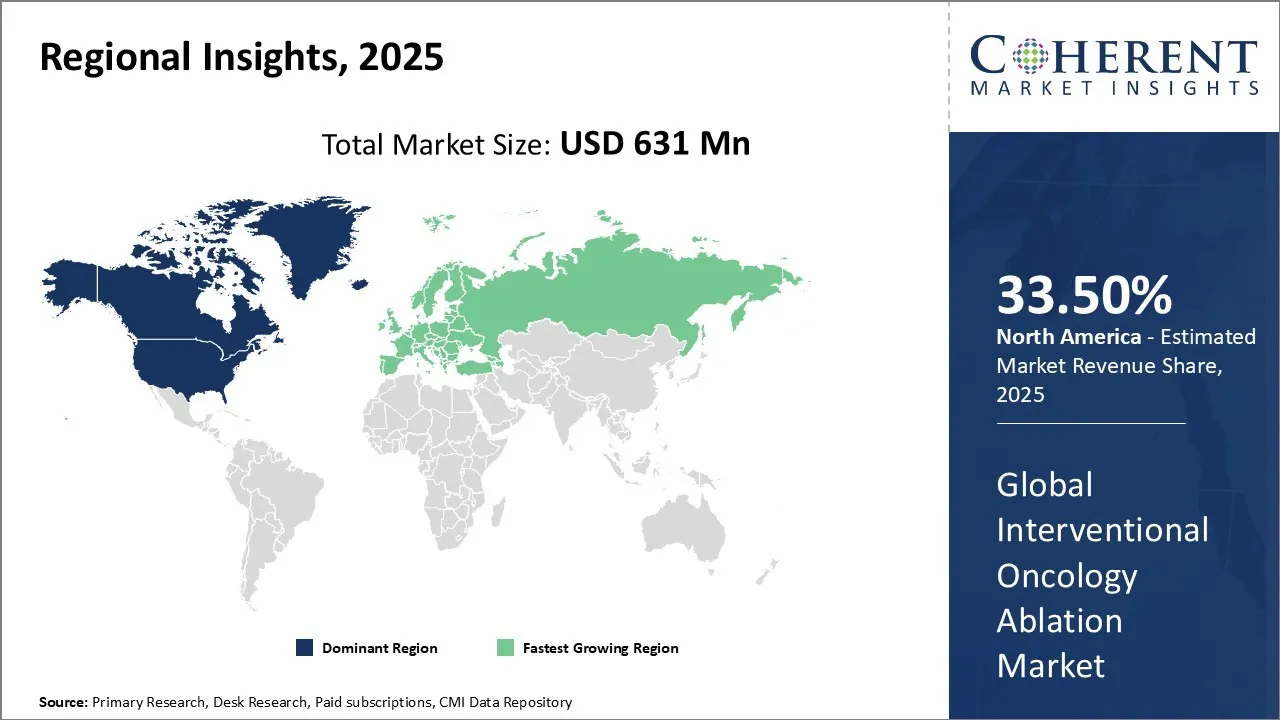
The Interventional Oncology Ablation Market is estimated to be valued at USD 631.0 Mn in 2025 and is expected to reach USD 1,131.5 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 8.7% from 2025 to 2032.
The market expands because more people develop cancer combined with better technology and increasing adoption of procedures that avoid extensive surgical approaches. RFA and MWA serve as popular ablation treatments for patients who cannot undergo surgery because of tumor size and location together with their general health condition. The widespread use of RFA persists because medical practitioners feel comfortable with familiar procedures although MWA demonstrates value in its rapid delivery of wide-reaching treatment fields. Treatment accuracy and safety receive improvement through the use of advanced imaging and navigation systems. MD Anderson and Charité Hospital lead developments in cancer research while advancing comprehensive ablation implementation within cancer care at their facilities.
|
Current Events |
Description and its impact |
|
Technological Advancements
|
|
|
Increasing Prevalence of Cancer |
|
|
Growing Preference for Minimally Invasive Procedures |
|
|
Increasing Health care Expenditure |
|
|
Collaborations and Partnerships |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
AI integration in the Interventional Oncology Ablation Market is transforming how cancer treatments are planned, executed, and monitored. The use of Artificial Intelligence (AI) in this sector is primarily focused on improving the precision, efficiency, and outcomes of ablation procedures. For instance, in September 2023, the Food and Drug Administration (FDA) granted 510(k) clearance for VisAble.IO (TechsoMed), an AI-powered software designed to enhance real-time imaging guidance during liver tumor ablation procedures. The software supports ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) to improve procedural accuracy.
In the U.S., Medicare provides reimbursement for radiofrequency ablation (RFA) for liver cancer treatment under the Medicare Physician Fee Schedule (MPFS). This reimbursement covers the costs of RFA procedures when used for the treatment of hepatocellular carcinoma (HCC) in patients who are not candidates for surgery or liver transplant. Medicare reimburses approximately $1,500 to $2,500 for an RFA procedure depending on the location and complexity of the treatment.
The Radio frequency ablation (RFA) segment leads as the principal part of the Interventional Oncology Ablation Market Share in 2025 with a projected share of more than 42.5%. The medical procedure finds extensive application in liver and renal tumor ablation because of its common handling mechanism and affordable cost. MD Anderson Cancer Center and other institutions have established universal protocols for performing RFA procedures on early-stage hepatocellular carcinoma. For instance, in July 2024, Stryker launched MultiGen 2 Radiofrequency (RF) Generator. This technology provides physicians with the efficiency, control and reliability they need when performing RF ablation, a minimally invasive procedure that can provide lasting relief to those suffering from facet joint pain.
Liver cancer, particularly hepatocellular carcinoma (HCC), remains the leading indication for ablation procedures due to its high prevalence globally and the effectiveness of techniques like radiofrequency ablation (RFA) and microwave ablation (MWA) in treating localized tumors. Also, liver cancer is a significant concern in the UK, with around 6,600 new cases diagnosed each year, which equates to approximately 18 new cases every day. Despite being the 17th most common cancer in the UK, liver cancer accounts for 2% of all new cancer cases in the country. It is particularly notable for its growing prevalence, with a steady rise in incidence rates over the past decade. Among females in the UK, liver cancer ranks as the 20th most common cancer, with approximately 2,200 new cases reported annually. The ablation methods offer advantages such as minimal recovery time, reduced complications, and the ability to treat patients who are not candidates for surgery. Technological advancements in imaging and device precision have further improved the success of these treatments, making them highly effective in targeting tumors, especially in challenging anatomical regions like the liver. While liver cancer dominates the market, other cancers such as lung, kidney, and pancreatic cancer also contribute to market demand.
Blood Hospitals and Clinics produce the largest amount of ablation procedures resulting in more than 60% of overall treatments. The healthcare facilities maintain the imaging systems that make ablation processes possible in precise ways. Several government hospitals throughout Germany together with Japan have built new oncology ablation facilities since the year 2020. Also, in February 2024, AIIMS Delhi launched a new AI solution for early cancer detection, developed in collaboration with the Centre for Development of Advanced Computing, Pune, under the Ministry of Electronics and Information Technology. The AI system utilizes deep learning models to analyze complex medical data with exceptional accuracy. These factors are further positively influencing the interventional oncology ablation market forecast.
To learn more about this report, Download Free Sample
The ablation sector in North America will rule the global market as it will hold a 33.5% stake in 2025 because of how the region quickly implements modern technologies and has supportive fee reimbursement systems and effective regulations. In July 2024, Varian, a company based in United States, received 510(k) clearance from the U.S. FDA for its IntelliBlate microwave ablation system, designed for soft tissue ablation. The system offers clinicians enhanced predictability, precision, and control during procedures, and is a key component of an integrated microwave ablation ecosystem. These factors are further escalating the interventional oncology ablation market demand in North America.
The European interventional oncology ablation market is experiencing robust growth, driven by several key factors. Technological advancements, such as the introduction of directional microwave ablation (MWA) and the Isolis cryoprobe, are enhancing procedural precision and expanding treatment options for challenging tumors. For example, the Philips' "Azurion" platform, combining advanced imaging with precise ablation tools, is improving treatment outcomes. The increasing preference for minimally invasive procedures, such as radiofrequency ablation (RFA) for liver cancer, offers benefits like reduced recovery times and lower complication rates. Additionally, favorable reimbursement policies in countries like France and Germany are improving patient access to these advanced treatments, further accelerating the interventional oncology ablation market growth.
Germany's interventional oncology ablation market is growing due to the increasing demand for minimally invasive surgery in cancer treatments and technological advancements. The use of advanced imaging techniques, such as CT and MRI-guided ablation, has significantly improved the accuracy and effectiveness of procedures. For example, the integration of tools like the IceCube cryoablation system has revolutionized the treatment of tumors by enabling precise, targeted destruction of cancerous tissue. Moreover, Germany’s healthcare system, which supports early cancer detection and offers favorable reimbursement for interventional oncology procedures, ensures greater access to these life-saving treatments. The rising number of cancer diagnoses in the country further drives this demand.
France's interventional oncology ablation market is experiencing significant growth, driven by several key factors. The French government has implemented policies to enhance access to advanced medical technologies, including tumor ablation procedures. Initiatives such as reimbursement schemes for minimally invasive treatment options and investment in healthcare infrastructure contribute to the growth of the tumor ablation market. Also, in May 2024, Clinical Laserthermia Systems AB (CLS) announced an exclusive distribution agreement with MTEC Company to market and sell the CLS TRANBERG® product portfolio in France. The partnership will focus on launching the TRANBERG system for ultrasound-guided focal laser ablation (FLA) of localized prostate cancer, utilizing temperature control technology. This is further propelling the interventional oncology ablation market revenue.
Precision navigation systems together with real-time image guidance represents the main trend shaping Interventional Oncology Ablation market growth. The effectiveness of tumor targeting receives assistance from 3D imaging systems that work together with robotic-needed placement devices to maintain high safety standards when targeting hard-to-reach anatomical areas. Non-operable tumor ablation procedures experience growing popularity as a trend in the market. More oncologists choose ablation as a treatment method for nonsurgical candidates to apply this technique toward lung and renal and bone tumor metastases. The expansion occurs because procedures yield improved results while patients stay in the hospital for reduced durations.
Multiple studies focus on investigating different options available in interventional oncology ablative procedures. The healthcare institutions of Cleveland Clinic together with Tata Memorial Hospital now publish joint reports to analyze outcomes from microwave ablation against radiofrequency ablation treatments in hepatic and pulmonary malignancies. Healthcare institutions are now designing new protocols to evaluate patients after interventional oncology procedures. Healthcare institutions have developed better protocols for post-ablation imaging follow-up with MRI along with PET-CT to check treatment success and track tumor regrowth thus advancing extended patient surveillance capabilities.
Research about sustainability between reusable and disposable ablation probes motivates manufacturers to create new ablation probe designs which support hospital waste reduction requirements and procurement policies.
The worldwide cancer incidence shows the most prominent effect on interventional oncology ablation market expansion. The Global Cancer Observatory predicts a substantial increase in new cancer cases up to 20 million every year through 2025 while liver cancer and lung cancer and kidney cancer will show the highest growth rates. The increasing case numbers in the population stimulates demand for tumor ablation treatments performed as localized minimally invasive procedures. Outpatient cancer care trends triggered the rapid implementation of ablation procedures in ambulatory surgical centers as well as smaller oncology clinics. The limited recovery time as well as associated minor complications of ablation make it a preferred choice in these treatment facilities over open surgical procedures, further accelerating the interventional oncology ablation market growth.
The Interventional Oncology Ablation Market presents its biggest possibilities when image-guided ablation needs expansion to low- and middle-income countries (LMICs). Ablative treatment methods provide LMICs with an affordable healthcare solution because they can be administered in facilities with basic equipment combined with outpatient facilities to address growing cancer cases.
The Brazilian along with Indian and South African governments initiate public funding for their cancer screening and localized treatment systems through purchases of microwave and radiofrequency ablation equipment. WHO’s global strategy to reduce premature cancer mortality through early detection and treatment supports these programs.
Through their investments the World Bank together with regional banks are helping construct healthcare infrastructure to build interventional oncology services in LMICs. The provided funding allows manufacturers to collaborate with public health systems for deploying mobile compact ablation units which function in areas without access to specialized imaging technology.
A set of multidimensional barriers exists to affect the growth trajectory of Interventional Oncology Ablation Market. Low- and middle-income nations face restricted use of advanced ablation procedures because of high equipment expense and insufficient specialized providers and improper imaging facilities. Various regulatory differences accompany reimbursement restrictions which impede widespread adoption of procedures particularly outside hospital facilities and outpatient care centers. Apart from this, lack of standard operating procedures and varying performance of medical devices between manufacturers causes compatibility issues between systems while decreasing physician trust in the system. The requirement of experienced operators who must undergo continuous training creates significant barriers when these professionals are scarce in particular markets.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 631.0 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.7% | 2032 Value Projection: | USD 1,131.5 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Boston Scientific Corporation, Johnson & Johnson, Medtronic Plc, Varian Medical Systems, Inc., EDAP TMS S.A., AngioDynamics, CASCINATION AG, Merit Medical Systems, Inc., STARmed Co., and Biomedical Srl. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients