Global lateral flow assay market is estimated to be valued at USD 11.05 Bn in 2025 and is expected to reach USD 15.55 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 5.0% from 2025 to 2032.
To learn more about this report, Download Free Sample
The lateral flow assay market is witnessing robust expansion globally, driven by the growing demand for rapid, point-of-care diagnostic solutions across healthcare, veterinary, food safety, and environmental testing. The versatility and ease of use of LFA devices, requiring minimal training or equipment—make them especially valuable in remote and resource-limited settings. Increasing prevalence of infectious diseases, rising awareness of early disease detection, and growing investments in decentralized testing infrastructure are key factors driving lateral flow assay market demand. Additionally, the demand is further reinforced by the surge in home-based testing kits for conditions like pregnancy, and influenza.
|
Current Event |
Description and Its Impact |
|
Global Infectious Disease Outbreaks |
|
|
Regulatory Framework Shifts |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of product type, kits and reagents segment is estimated to contribute the highest market share of 55.5% in 2025, owing to their reliability and ease of use. Lateral flow assays based on kits and reagents provide consistent and standardized results without requiring specialized equipment or training to perform. The disposable nature of test strips and cassettes included in kits eliminates risks of contamination between tests. This reliability and convenience make kits and reagents well suited for point-of-care testing applications. Kits contain pre-measured and pre-packaged reagents along with all materials needed to complete the assay, simplifying the testing process. This simplification expands the range of settings where lateral flow tests can be administered. Kits empower healthcare workers and patients themselves to conduct tests in community healthcare centers, hospitals, homes, and even in field conditions. Their user-friendly design promotes adherence to testing protocols. Reagents form the key components of lateral flow strips and devices. Stable and optimized reagents maximize test sensitivity and specificity.
Moreover, leading manufacturers leverage extensive research and development to formulate high performing reagents. Continuous refinement of reagents assists kit makers in enhancing lateral flow technologies. New innovations in labeling, detection, and stabilization of reagents further boosts reliability. The product lifecycle of kits also depends on the stability of constituent reagents during storage and use. Kits provide a standardized and simplified solution for lateral flow testing. Their user-friendliness and ease of access drives the segment growth.
In June 2025, Abingdon Health plc has entered a strategic co-development and commercialization agreement with Netherlands-based Okos Diagnostics to launch a rapid avian flu (H5N1) lateral flow assay (LFA) Kit in New Okos Partnership, targeting both bovine and human applications. Such product developments are accelerating the lateral flow assay market growth.
In terms of technique, multiplex detection assay segment is estimated to contribute the highest market share of 30.5% in 2025, owing to its ability to detect multiple analytes simultaneously. Unlike qualitative assays indicating only presence or absence, multiplex detection allows quantitative analysis of several targets in one reaction well. This multiplexing capability addresses a key limitation of conventional lateral flow tests, which typically only detect a single analyte. It empowers developers to design compact multi-parameter tests. This has applications in screening for co-infections like respiratory panels, cardiac marker panels, allergy tests, as well as research use. The possibility to detect a panel of biomarkers correlated to a disease enhances accuracy of diagnosis. It enables risk stratification, monitoring of disease progression, and guiding treatment decisions. Monitoring response to therapies requires analysis of multiple parameters. Furthermore, multiplexing conserves sample volume requirements and produces results faster than running individual single-plex tests serially.
In May 2025, a research team launched Cas12a cis-cleavage mediated lateral flow assay (cc‑LFA) that enables highly specific and multiplexed nucleic acid detection. By integrating a novel “double‑key” mechanism—CRISPR‑Cas12a cis-cleavage plus invasive hybridization—the test achieves single‑base resolution with >90% sensitivity and 100% specificity across multiple respiratory pathogens and nine high‑risk HPV subtypes.
In terms of application, clinical testing segment is estimated to contribute the highest market share of 35.5% in 2025, owing to the widespread use of lateral flow technology across various medical diagnostic applications. Currently, clinical testing constitutes more than half of the total lateral flow assay market. Lateral flow assays find diverse applications across various specialties like infectious disease testing, cardiac markers, toxicology, fertility, and others. Their ease of use and rapid results makes them suitable for point-of-care testing in physician offices and hospital settings. Furthermore, self-testing is growing for applications like pregnancy, and fertility, using home-use lateral flow kits. The ability to rapidly diagnose conditions influences patient management decisions. It allows proper isolation of infectious patients. Rapid results in emergency rooms facilitate quick triaging of patients. Bedside testing sustains continuous monitoring of patients. Lateral flow assays prove especially valuable in resource-limited primary healthcare centers in developing countries for basic screening and diagnosis.
In March 2025, Retailers like Boots UK began selling finger‑prick kits to measure iron, cholesterol, vitamin D, and influenza. Clinical trials are underway for stroke and sepsis detection in emergency and home settings, using blood and novel samples such as sweat, water, and even pet vomit. Such developments are contributing to the lateral flow assay market share.
In terms of end user, the hospitals & Clinics segment dominate the lateral flow assay market in 2025 with largest share, due to their central role in primary diagnostics and immediate patient care. Hospitals and clinics have higher testing volumes, faster turnaround requirements, and more access to point-of-care testing kits. With rising demand for rapid diagnostics in emergency settings and outpatient care, lateral flow assays have become a preferred choice in these facilities for quick, on-the-spot results, particularly for infectious diseases, cardiac markers, and pregnancy testing.
To learn more about this report, Download Free Sample
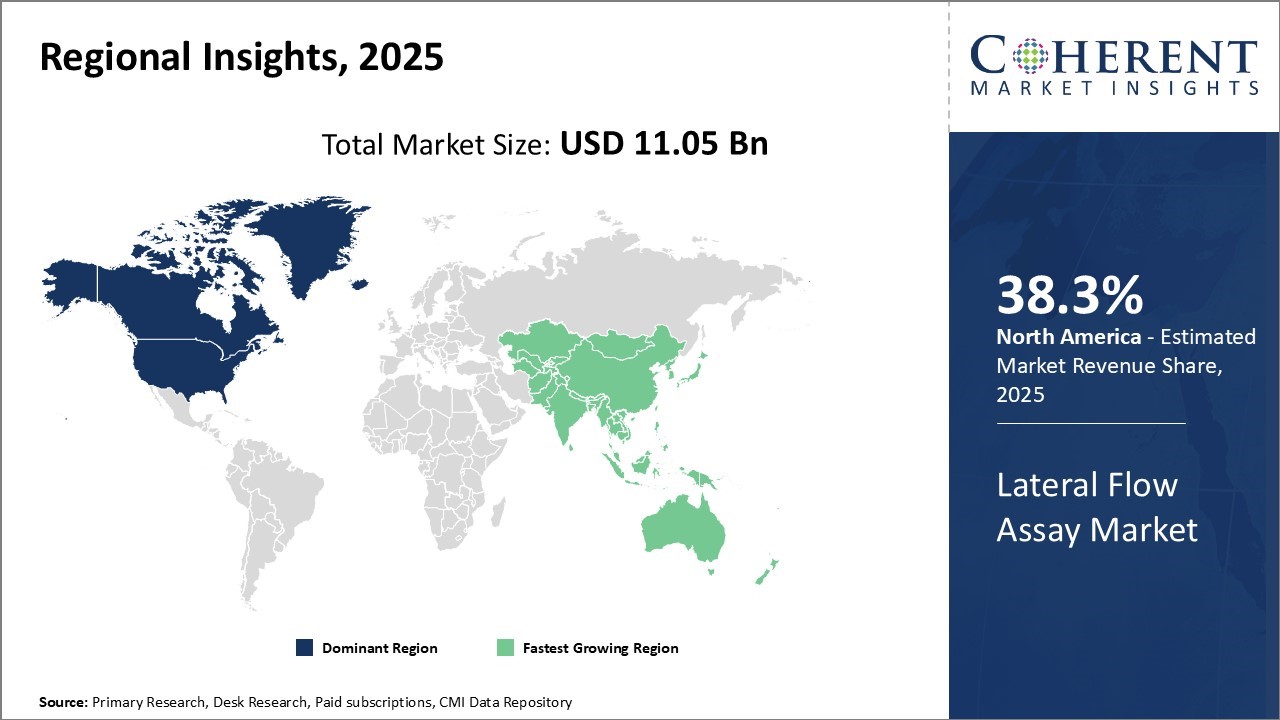
North America currently dominates the global lateral flow assay market with an estimated market share of 38.3% in 2025, led primarily by the U.S. With huge presence of leading diagnostics companies and robust healthcare infrastructure, the U.S. lateral flow assay market has grown steadily over the years. The region has established distribution channels and reimbursement structures in place, allowing for smooth adoption of new lateral flow technologies. High per capita healthcare spending provides lucrative growth opportunities for manufacturers. However, industry experts note pricing pressures due to payor initiatives like reference pricing can impact profits.
In July 2025, VolitionRx, a multi-national epigenetics company, engages in the development of blood tests to help diagnose and monitor a range of cancers, has unveiled a lateral flow device capable of quantifying nucleosomes in whole venous blood within minutes, validated in a blinded SUMMIT study involving 25 ICU and emergency department patients.
Asia Pacific region has emerged as the fastest-growing market for lateral flow assays globally. Rapid economic development and rising affluence have boosted healthcare investments across developing nations like India and China. This has increased accessibility to diagnostic testing. Furthermore, a burgeoning geriatric population base that is more prone to chronic diseases boosts demand. With growing health awareness, preventive care has gained prominence, boding well for products detecting diseases early. Favorable regulations by regulatory bodies to fast-track new products are encouraging Western companies to tap into untapped markets. However, manufacturers will need to optimize supply chains and pricing strategies to succeed, given regional cost differences.
The United States is a leading market in the Lateral Flow Assay Market demand due to its high burden of infectious and chronic diseases, strong point-of-care testing adoption, and robust diagnostic industry. The FDA has approved multiple lateral flow-based kits for flu, and RSV, including over-the-counter tests, boosting home diagnostics. Companies like Abbott and QuidelOrtho continue to launch combo test kits, supporting widespread use. Also, MIT chemists from the Swager Lab have developed a wireless electronic lateral flow assay that uses electronic polymers instead of colored lines, allowing for quantitative detection of biomarkers. Apart from this, government support and insurance coverage further encourage adoption, making the U.S. a key driver of lateral flow assay growth.
India’s demand for lateral flow assays is rising due to its large population, rural healthcare expansion, and government health programs. Under the National Health Mission, rapid tests for malaria, tuberculosis, HIV, and pregnancy are widely used in rural clinics. These tests are ideal for low-resource settings due to their speed and simplicity. For example, malaria RDTs and pregnancy kits are commonly used by ASHA workers for early detection. Local manufacturing and government efforts to expand diagnostic access continue to support lateral flow assay market demand across the country.
China is witnessing strong lateral flow assay market due to rapid healthcare expansion and focus on early disease detection. The Chinese CDC has adopted LFAs in large-scale community screening programs for infections like hepatitis and HIV, enabling faster diagnosis in rural areas. Local manufacturers such as Wondfo and Innovita are scaling production to meet both domestic and export needs. These initiatives, supported by growing public health awareness and infrastructure investment, are driving market growth.
To learn more about this report, Download Free Sample
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 11.05 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.0% | 2032 Value Projection: | USD 15.55 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
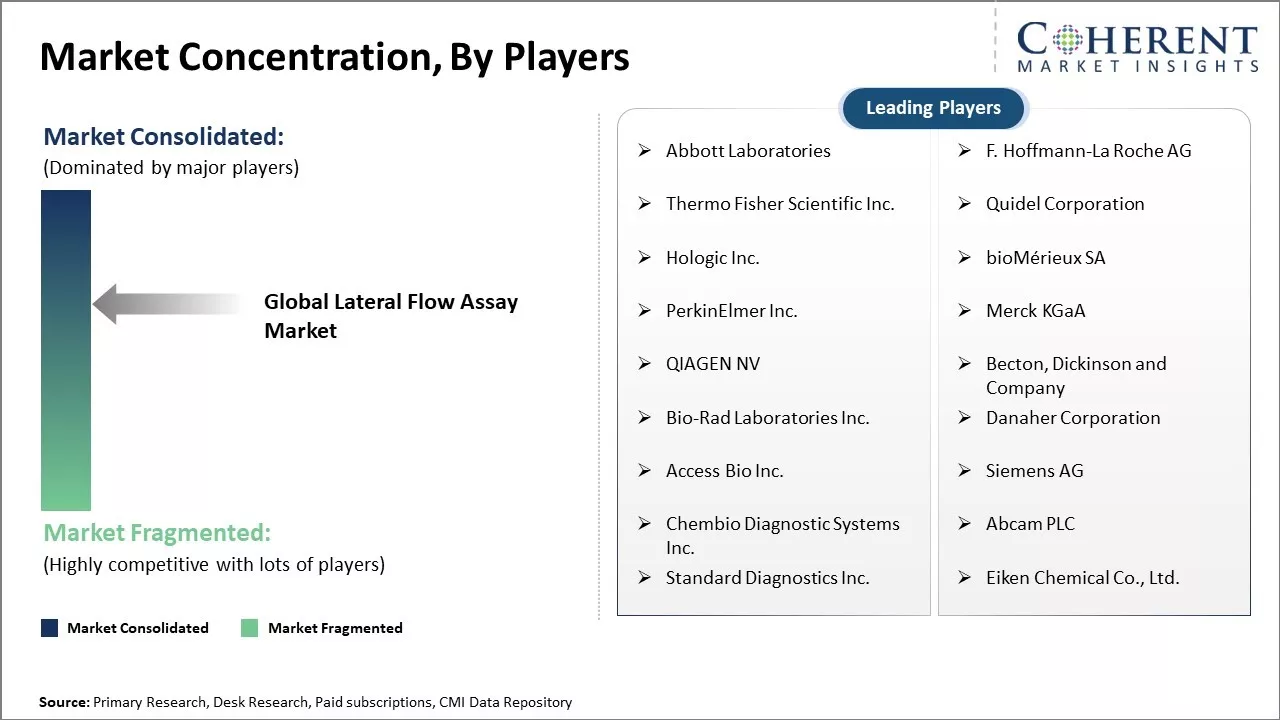
Abbott Laboratories, F. Hoffmann-La Roche AG, Thermo Fisher Scientific Inc., Quidel Corporation, Hologic Inc., bioMérieux SA, PerkinElmer Inc., Merck KGaA, QIAGEN NV, Becton, Dickinson and Company, Bio-Rad Laboratories Inc., Danaher Corporation, Access Bio Inc., Siemens AG, Chembio Diagnostic Systems Inc., Abcam PLC, Standard Diagnostics Inc., Eiken Chemical Co., Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
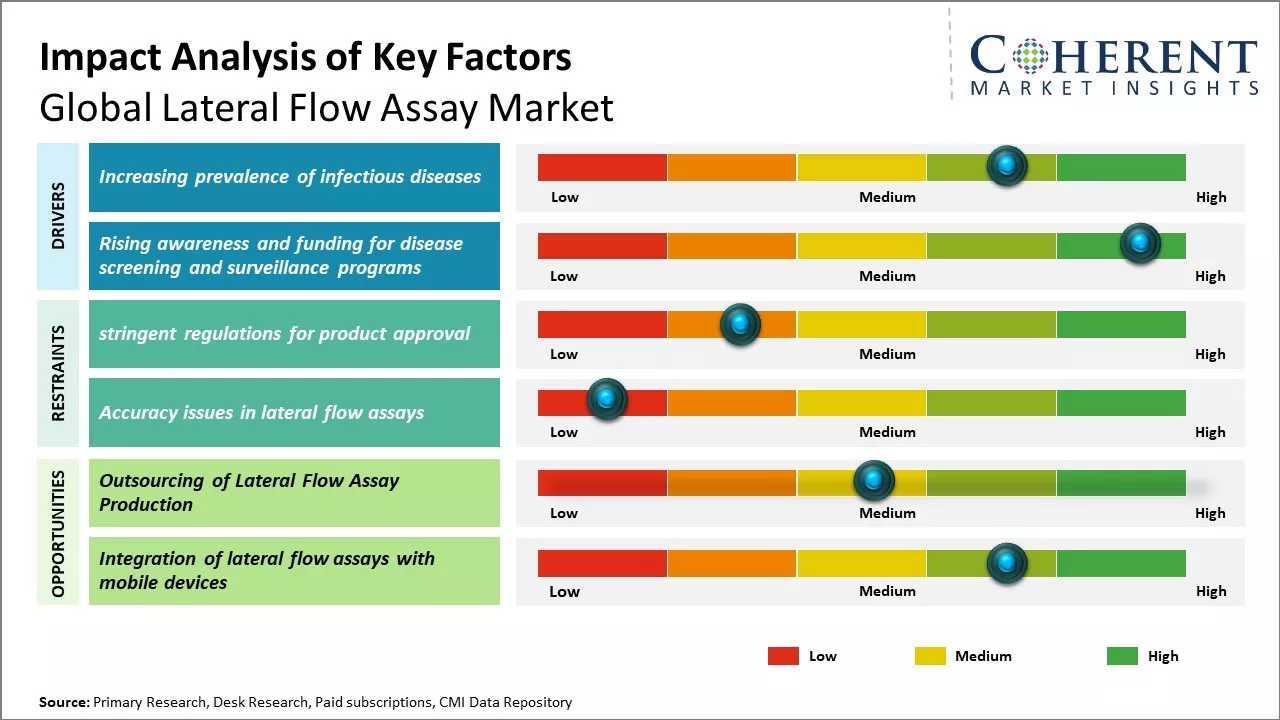
Rising prevalence of infectious diseases across the globe can drive the global lateral flow assay market growth. There has been increase in infectious diseases due to various factors like climate change, increased globalization, higher antimicrobial resistance, and evolving epidemiology of existing and new pathogens. As per the World Health Organization (WHO), infectious diseases account for around 17 million deaths globally every year, which is approximately one-third of all deaths worldwide. The occurrence of new strains of existing diseases like, viral hemorrhagic fevers, and vector-borne illnesses due to factors like climate change, can exacerbate the situation. Growing threat posed by infectious diseases has boosted demand for rapid, cost-effective, and easy-to-use screening and diagnostic tests. Lateral flow assay tests meet all these requirements, and this leads to their increased adoption rate. Their ability to provide quick results without the need for laboratory infrastructure or specialized technicians has fueled their popularity in point-of-care testing, further contributing to the lateral flow assay market share.
Rising awareness about the importance of disease screening and surveillance globally can drive the market growth. Both developing and developed countries focuses on implementation of national screening programs for priority health issues to achieve early detection and treatment. This boosts demand for point-of-care testing devices. Moreover, there has been increase in government and private sector funding for infectious disease control, maternal and child healthcare programs. All these screening initiatives rely heavily on rapid, portable assays to decentralize testing and reach remote populations. Lateral flow assays are used in large surveillance programs due to their applicability in resource-limited field conditions and ability to provide timely results. These assays have been widely used in HIV, malaria and tuberculosis prevalence studies across Africa and Asia. Their usage grows further with more funding directed at expanding testing and vaccination coverage for communicable and non-communicable diseases. The portability and ease of use of lateral flow assays makes them a preferred technology for field healthcare camps, refugee camps and other temporary testing sites.
The lateral flow assay market value is undergoing a meaningful evolution, driven by a systemic realignment of diagnostic priorities across global healthcare ecosystems.
One of the most compelling signals is the growing investment in multiplexed LFA platforms, particularly in oncology and cardiology diagnostics. Companies like QuidelOrtho and Abingdon Health are advancing beyond traditional single-analyte strips to enable simultaneous detection of multiple biomarkers with sub-nanogram sensitivity. This reflects a market maturity that sees LFAs not just as basic screening tools, but as integrated diagnostic systems capable of replacing or augmenting laboratory-based assays in decentralized settings.
Furthermore, the demand landscape is becoming more stratified. High-income countries are no longer driving growth solely through scale, but through innovation in lateral flow formats such as smartphone-integrated readers and AI-enhanced interpretation algorithms. For example, firms in Western Europe are collaborating with digital health platforms to offer real-time clinical decision support based on LFA outcomes, targeting chronic disease management and remote patient monitoring.
Meanwhile, in low- and middle-income countries (LMICs), organizations like FIND and the Bill & Melinda Gates Foundation are channeling resources into LFAs for tuberculosis and antimicrobial resistance (AMR) detection. The World Health Organization estimates that up to 70% of diagnostics in LMICs are delivered through rapid tests, making the LFA the de facto infrastructure for early-stage intervention. Yet, despite significant advancements, the issue of diagnostic accuracy in high-temperature and high-humidity environments remains unresolved in many tropical regions.
*Definition: Global Lateral Flow Assay Market involves testing techniques used to detect the presence and concentration of analytes in samples without the need for specialized and expensive equipment. Lateral flow assays use commercial chromatography principles to separate analytes based on size and affinity to determine results either visually or with a meter. They offer rapid, low-cost testing solutions for various applications such as clinical diagnostics, food safety testing, and environmental monitoring on a global scale.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients