Relapsed or Refractory Diffuse Large B-cell Lymphoma Market is estimated to be valued at USD 1,610.0 Mn in 2025 and is expected to reach USD 2,161.8 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 4.3% from 2025 to 2032.
Analysts’ Views on Global Relapsed or Refractory Diffuse Large B Cell Lymphoma Market:
Increasing relapse and refractory cases of diffuse large B cell lymphoma (DLBCL) are expected to drive the global relapsed or refractory diffuse large B cell lymphoma market growth over the forecast period. This is expected to boost demand for innovative treatment options and contribute to the overall growth of the market. Furthermore, growing prevalence of DLBCL globally is also a significant factor contributing to market growth. Rising number of diagnosed cases necessitates the development of more effective therapies, thereby, boosting demand for innovative treatment options in the market. Advancements in medical research and technology enables discovery of novel therapeutic approaches for DLBCL, and this boosts demand for innovative treatment options. Moreover, increasing investments by pharmaceutical companies and government organizations for research and development activities related to DLBCL is expected to drive the market growth by facilitating the introduction of new and improved treatment options.
Figure 1. Global Relapsed or Refractory Diffuse Large B Cell Lymphoma Market Share (%), By Drug Type, 2025
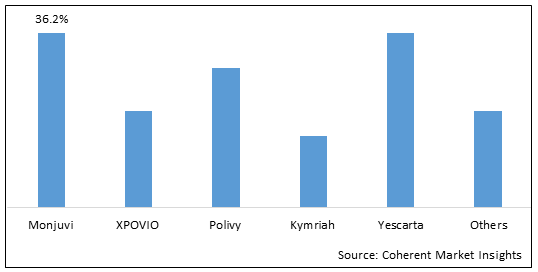
To learn more about this report, Download Free Sample
Global Relapsed or Refractory Diffuse Large B Cell Lymphoma Market– Drivers
Figure 2. Global Relapsed or Refractory Diffuse Large B Cell Lymphoma Market Share(%), By Region, 2025
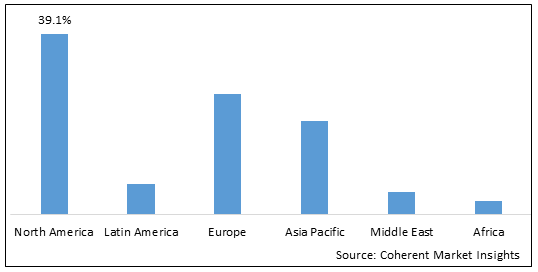
To learn more about this report, Download Free Sample
Global Relapsed or Refractory Diffuse Large B Cell Lymphoma Market- Regional Analysis
Global Relapsed or Refractory Diffuse Large B Cell Lymphoma Market– Impact of Coronavirus (COVID-19) Pandemic
Global Relapsed or Refractory Diffuse Large B Cell Lymphoma Market Segmentation:
Global relapsed or refractory diffuse large B cell lymphoma market is segmented into drug type, distribution channel, and region.
Global Relapsed or Refractory Diffuse Large B Cell Lymphoma Market- Cross Sectional Analysis:
Key players are making relapsed or refractory diffuse large B cell lymphoma with combination of drug which is expected to drive the relapsed or refractory diffuse large B cell lymphoma market in North America region. For instance, in April 2021, Eagle Pharmaceuticals, Inc., a pharmaceutical company with research and development, clinical, manufacturing and commercial expertise, announced that TREAKISYM ready-to-dilute (“RTD”) (bendamustine hydrochloride 120 mg/m2) liquid formulation had been approved for a new indication in combination with rituximab (“BR therapy”) as treatment for relapsed or refractory diffuse large B-cell lymphoma (“r/r DLBCL”) by the Pharmaceuticals and Medical Devices Agency (“PMDA”) in Japan.
Relapsed or Refractory Diffuse Large B Cell Lymphoma Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,610.0 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.3% | 2032 Value Projection: | USD 2,161.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
MorphoSys U.S. Inc., Bristol-Myers Squibb Company, Karyopharm Therapeutics, Hoffmann-La Roche AG, Merck & Co., Inc., Gilead Sciences, Inc., Novartis AG, Regeneron Pharmaceuticals, Cellular Biomedicine Group Inc., Genmab A/S, Incyte, AbbVie Inc., Janssen Biotech, Inc., Pfizer Inc., IMV Inc., Overland Pharmaceuticals (CY) Inc., ADC Therapeutics SA, Eagle Pharmaceuticals, Inc., and Adaptive Biotechnologies Corporation |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Relapsed or Refractory Diffuse Large B Cell Lymphoma Market: Key Developments
Global Relapsed or Refractory Diffuse Large B Cell Lymphoma Market: Key Trends
Global Relapsed or Refractory Diffuse Large B Cell Lymphoma Market: Restraint
Global Relapsed or Refractory Diffuse Large B Cell Lymphoma Market - Key Players
Major players operating in the global relapsed or refractory diffuse large B cell lymphoma market include MorphoSys U.S. Inc., Bristol Myers Squibb, Karyopharm Therapeutics, Hoffmann-La Roche AG, Merck & Co., Inc., Gilead Sciences, Inc., Novartis AG, Regeneron Pharmaceuticals, Cellular Biomedicine Group Inc., Genmab A/S, Incyte, AbbVie Inc., Janssen Biotech, Inc., Pfizer Inc., IMV Inc., Overland Pharmaceuticals (CY) Inc., ADC Therapeutics SA, Eagle Pharmaceuticals, Inc., and Adaptive Biotechnologies Corporation.
*Definition: Diffuse large B-cell lymphoma (DLBCL) is a fast-growing blood cancer. Healthcare providers typically treat this condition with a combination of cancer drugs. The combined drugs often eliminate DLBCL signs and symptoms and cure the condition. Medical researchers are studying different treatments for DLBCL that don’t respond to treatment or regenerate.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients