Specialty injectable market is estimated to be valued at USD 62.62 Bn in 2025 and is expected to reach USD 122.10 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 10.0% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Specialty injectable market has been witnessing positive growth trends due to rising prevalence of chronic diseases along with increasing demand for self-injectable pharmaceutical products. Growth in biologics drug delivery and increasing demand for targeted drug delivery systems can also aid the market expansion. Patent expiration of major biologic drugs also presents opportunities for biosimilar products, and this drives the market growth. Complex manufacturing process and robust regulatory procedures incur significant costs, posing challenges to market players.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
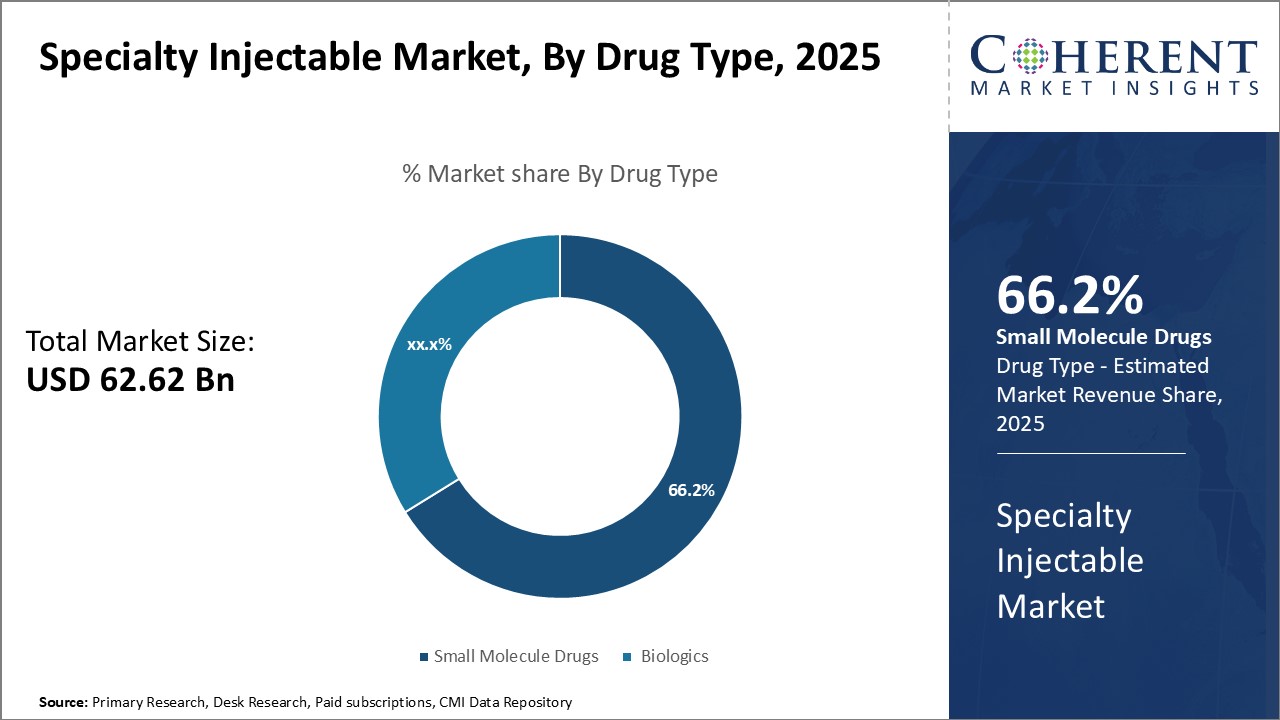
Insights, By Drug Type- Growth of Biosimilars Pushes Small Molecule Drugs Segment
In terms of drug type, small molecule drugs segment is estimated to contribute the highest market share of 65.6% in 2025, owing to growth of biosimilars. Small molecule drugs offer cost savings as compared to biologics and have well-established manufacturing processes. The development and approval of lower-cost biosimilars has boosted the small molecule drugs segment.
Insights, By Application- Rising Cancer Incidence Fuels Oncology Applications
In terms of application, oncology segment is estimated to contribute the highest market share of 49.8% in 2025, due to rising global incidence of cancer and growing reliance on targeted drug therapies. In 2021, according to the WHO, cancer is among the leading causes of death worldwide with nearly 10 million deaths in 2020.
Insights, By Distribution Channel- Role of Hospitals in Complex Therapies Boosts Hospital Pharmacies
In terms of distribution channel, hospital pharmacies segment is estimated to contribute the highest market share of 54.7% in 2025, due to growing role of hospitals in administering complex specialty injectable medications. Expanding armamentarium of innovative injectable drugs and emphasis on precision medicine drives greater complexity in administrations.
Need a Different Region or Segment? Download Free Sample
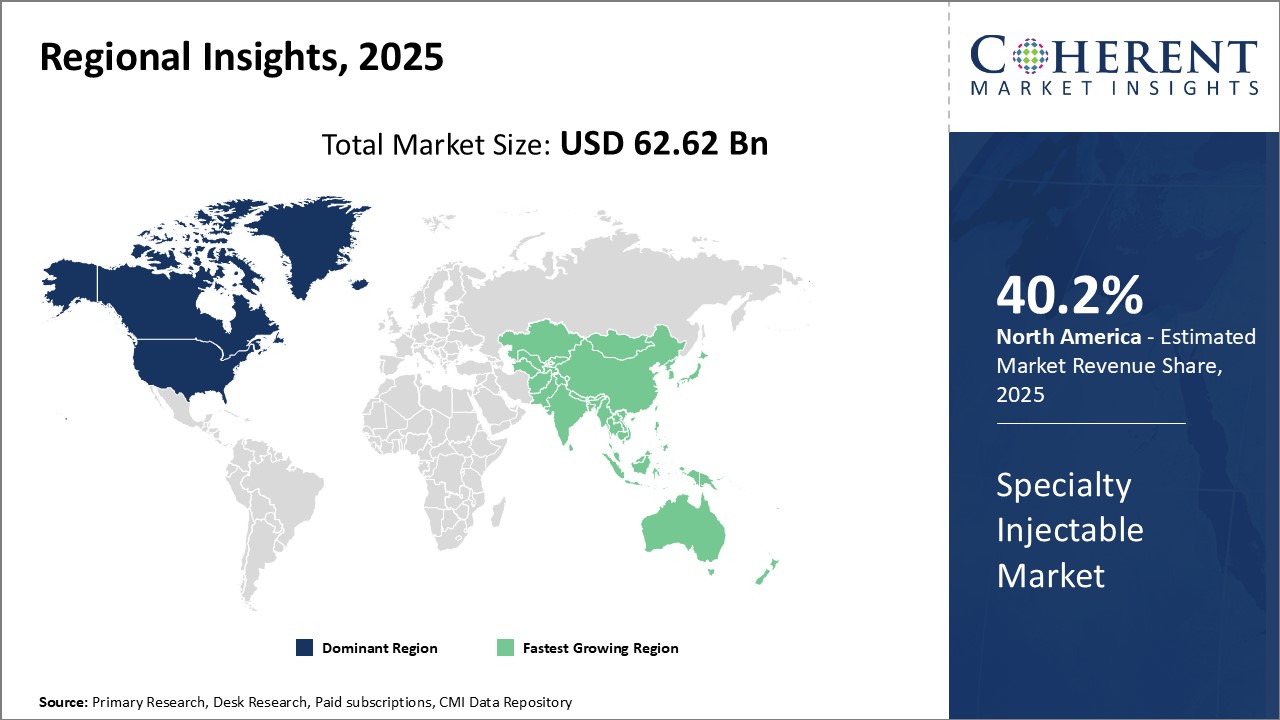
Dominating Region- North America
North America is expected to dominate the specialty injectable industry, with an estimated market share of 40.2% in 2025, due to strong presence of leading pharmaceutical companies, widespread healthcare infrastructure, and favorable government policies that encourage research and development.
Fastest-Growing Region- Asia Pacific
Asia Pacific region exhibits the fastest growth in specialty injectable industry, with an estimated market share of 19.2% in 2025, due to large patient population, rising healthcare expenditure, and increasing focus on quality healthcare.
Specialty Injectable Market Outlook for Key Countries
Investments for drug development in the U.S.
Specialty injectable industry in the U.S. is thriving due to substantial investments in innovative drug formulations. Leading companies like Pfizer, Inc. dominate this sector, utilizing their extensive product portfolios to meet diverse healthcare needs and rapidly adapt to emerging trends, thus, driving growth and enhancing patient outcomes.
Rising Demand for Specialty Medications in Canada
Canada specialty injectable industry growth is driven by growing need for specialty medications to treat chronic, rare, and complex conditions such as cancer, hepatitis C, and autoimmune disorders. Canada's aging demographic contributes to higher prevalence of health issues that require specialty medications, thus, boosting demand for injectable therapies.
Government initiatives in China
China's specialty injectable industry is experiencing robust growth due to government initiatives aimed at modernizing the healthcare system. Local leaders are enhancing their capabilities to meet the rising demand for innovative therapies, ensuring improved access and affordability of specialty injectables for patients across the nation.
Japan’s increasing research and development activities
Continuous innovations in injectable formulations and delivery systems are enhancing treatment outcomes, making specialty injectable more appealing to healthcare providers and patients alike. Regulatory bodies are increasingly supportive of innovative injectable formulations, streamlining approval processes for new delivery systems and formulations.
Favorable population demographics in India
India's specialty injectable industry can witness growth due to favorable population demographics and initiatives to enhance local manufacturing. Dr. Reddy's Laboratories Ltd. is a key market playeraiming to leverage these trends to meet increasing healthcare demands and improve access to innovative injectable therapies across the country.
Get actionable strategies to beat competition: Download Free Sample
Top Strategies Followed by Specialty Injectable Market Players
Emerging Startups in the Specialty Injectable Market
Key Takeaways from Analyst
Specialty Injectable Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 62.62 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.0% | 2032 Value Projection: | USD 122.10 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
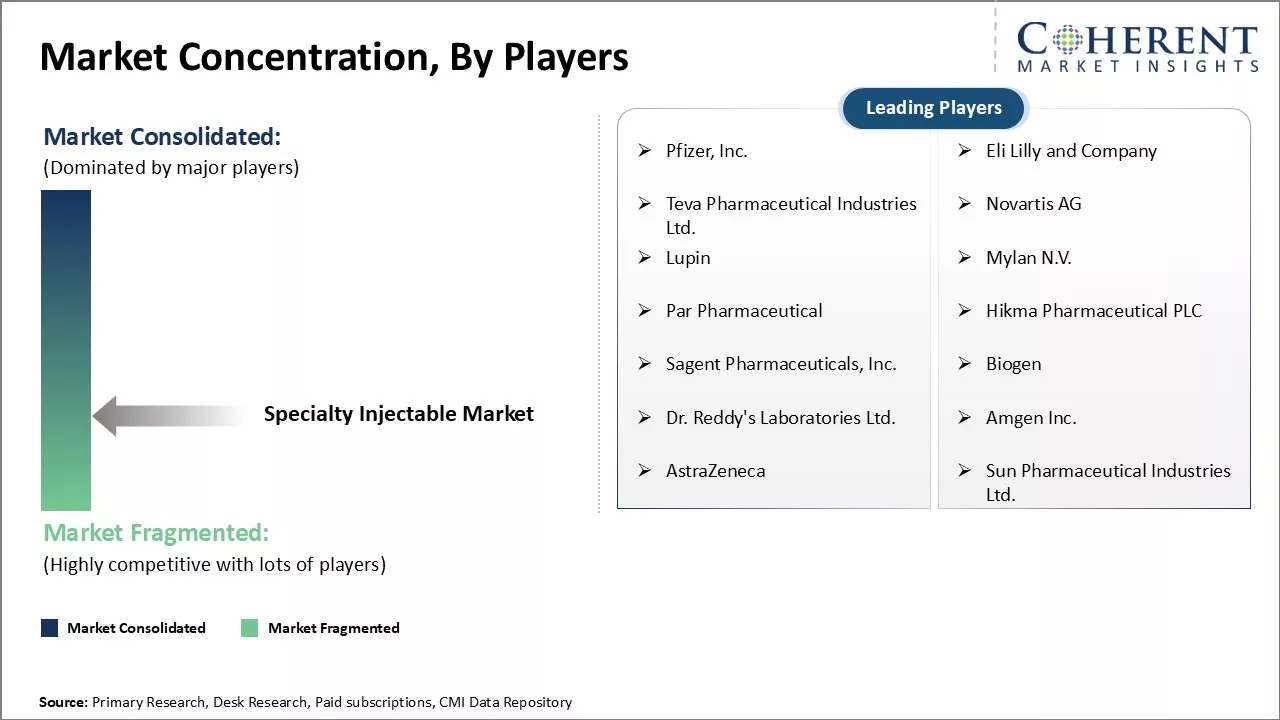
Pfizer, Inc., Eli Lilly and Company, Teva Pharmaceutical Industries Ltd., Novartis AG, Lupin, Mylan N.V., Par Pharmaceutical, Hikma Pharmaceutical PLC, Sagent Pharmaceuticals, Inc., Biogen, Dr. Reddy's Laboratories Ltd., Amgen Inc., AstraZeneca, Sun Pharmaceutical Industries Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
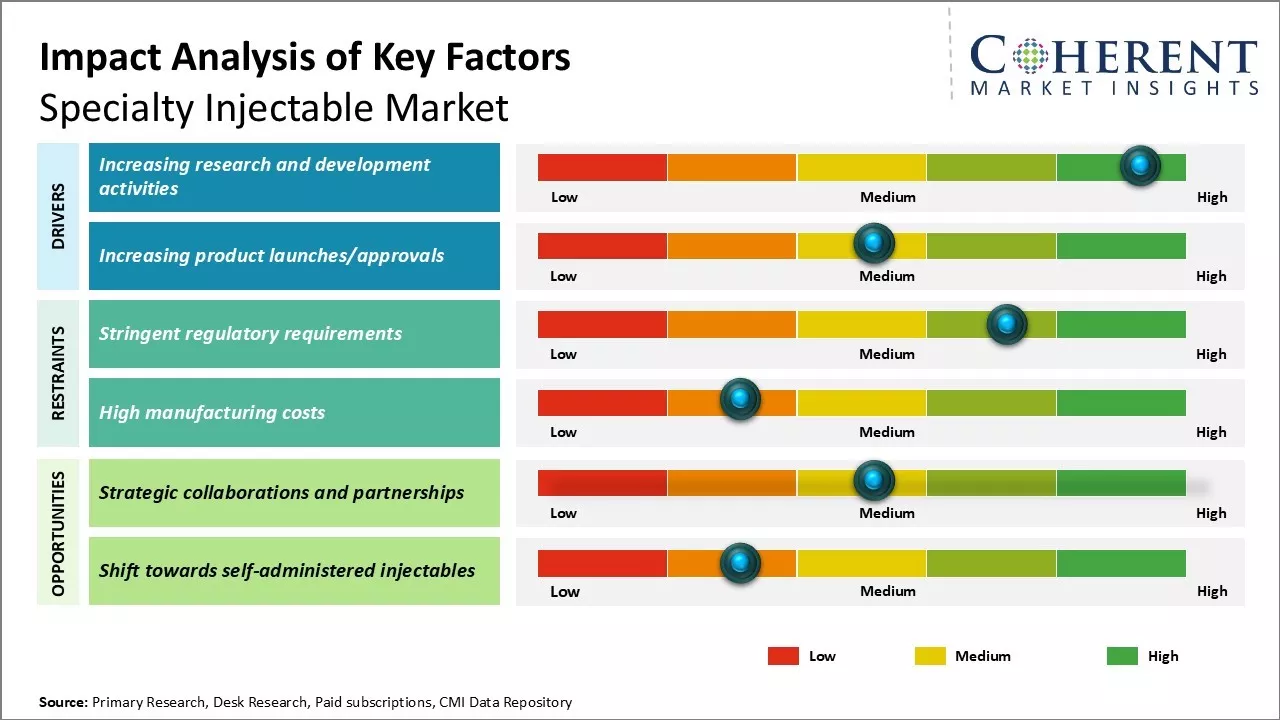
Market Driver - Increasing product launches/approvals
Increasing product launches/approvals can drive the market growth over the forecast period. For instance, on April 16, 2024, Amneal Pharmaceuticals, Inc., a pharmaceutical company, announced the launch of PEMRYDI RTU, the first and only ready-to-use presentation of pemetrexed for injection. This product does not require reconstitution, dilution, or refrigeration, which is different than other versions of pemetrexed for injection.
Market Challenge - Stringent regulatory requirements
Regulatory authorities like the U.S. Food and Drug Administration and European Medicine Agency meticulously oversee every phase of drug development and manufacturing. Obtaining approvals involves extensive clinical testing and documentation of quality, safety, and efficacy. Additional requirements, such as post-marketing surveillance, increase compliance burdens, necessitating significant investments that heighten costs and financial risks. Lengthy review periods and uncertain approval outcomes further complicate timelines, making compliance issues potentially detrimental to timely market entry and operational continuity.
Market Opportunity - Strategic collaborations and partnerships
Strategic collaborations and partnerships are anticipated to significantly enhance growth opportunities for key players in the specialty injectable market. These alliances can facilitate expanded market access and improved capabilities, driving lucrative growth in this sector as demand for specialty injectable drugs continues to rise due to increasing chronic diseases and advancements in healthcare technologies.
What growth in specialty injectable industry mean for different stakeholders?
Specialty injectable industry has multiple players with varied designations and offers multiple opportunities based on their scope of operations.
|
Key Pharmaceutical Stakeholder |
Opportunities Due to Specialty Injectable Industry Growth |
|
Retail Pharmacies |
Expansion of product offerings to include new drugs and personalized medicine solutions, enhancing customer care and market reach. |
|
Chemical Suppliers |
Growth in demand for specialty chemicals used in drug synthesis, including organic intermediates, catalysts, and reagents. |
|
Pharmaceutical Companies |
Expansion of product pipelines with new drug discoveries, biologics, and biosimilars, capitalizing on growing global healthcare needs. |
|
Contract Research Organizations (CROs) |
Increased outsourcing of clinical trials and drug development, offering opportunities for growth and long-term partnerships. |
|
Contract Manufacturing Organizations (CMOs) |
Growing demand for scalable manufacturing solutions, including biologics production and complex drug formulations. |
|
Diagnostic Equipment Manufacturers |
Expanded markets for diagnostic tools and devices that support personalized medicine and early disease detection. |
|
Healthcare Providers |
New treatment options and innovative therapies, improving patient care and expanding healthcare services. |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients