Alk Positive Lung Cancer Treatment Market Size and Forecast – 2025 – 2032
The Global Alk Positive Lung Cancer Treatment Market size is estimated to be valued at USD 4.7 billion in 2025 and is expected to reach USD 9.8 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 11.2% from 2025 to 2032.
Global Alk Positive Lung Cancer Treatment Market Overview
ALK-positive lung cancer treatments comprise targeted therapies that inhibit abnormal anaplastic lymphoma kinase (ALK) gene fusion activity found in a subset of non-small cell lung cancers (NSCLC). These treatments primarily include small-molecule tyrosine kinase inhibitors (TKIs) that block ALK signaling pathways, preventing tumor cell proliferation and metastasis. Products such as crizotinib, ceritinib, alectinib, and lorlatinib have demonstrated high specificity and durable response rates, reshaping the therapeutic landscape for advanced-stage patients.
Modern formulations are optimized for blood-brain barrier penetration, addressing central nervous system metastases common in ALK-positive patients. Advances in next-generation sequencing (NGS) diagnostics have improved companion testing accuracy, ensuring precise drug-patient matching. Continuous innovation in resistance mutation targeting and combination regimens is expanding therapeutic efficacy, making ALK inhibitors a core component of precision oncology treatment strategies.
Key Takeaways
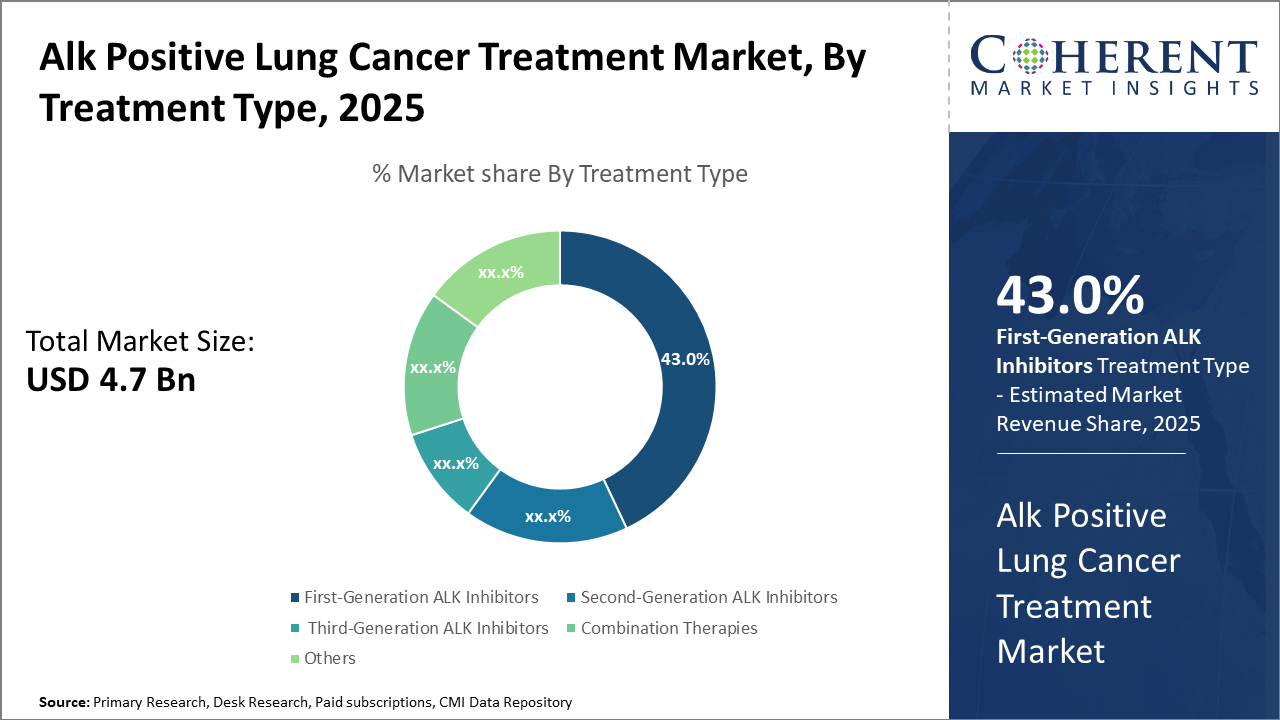
The First-Generation ALK Inhibitors segment holds the largest market share at 43%, driven by established clinical use and cost-effectiveness.
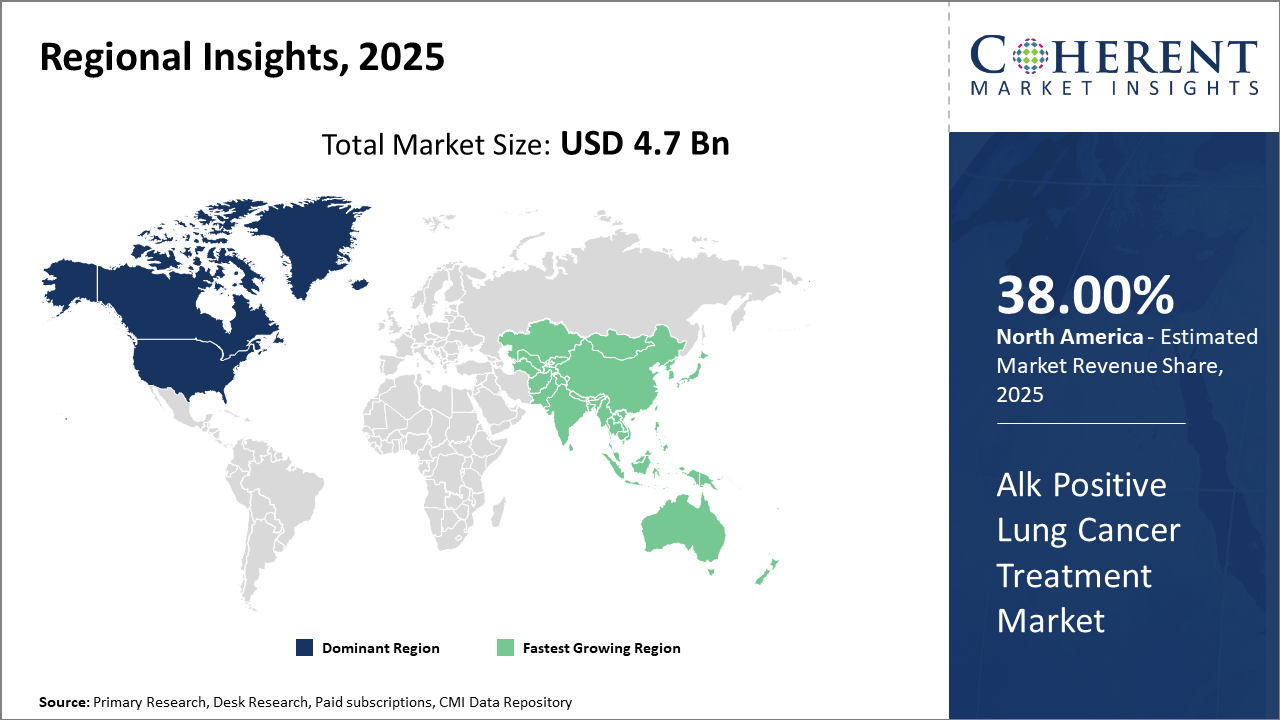
North America dominates the market share, accounting for over 38% of the Alk Positive Lung Cancer Treatment market, supported by advanced healthcare infrastructure and reimbursement policies.
Asia Pacific is the fastest-growing region with a projected CAGR exceeding 13%, propelled by increasing healthcare investments and rising patient awareness in emerging economies.
Alk Positive Lung Cancer Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Alk Positive Lung Cancer Treatment Market Insights, By Treatment Type
First-generation ALK Inhibitors dominate the market share, owing to their established clinical efficacy, widespread adoption, and comparatively lower cost, securing over 43% market share. They continue to act as the front-line treatment in many clinical protocols despite new entrants. The fastest growing subsegment is the Second-Generation ALK Inhibitors, propelled by their ability to overcome resistance seen with first-generation treatments, leading to improved patient survival rates and longer progression-free intervals. These variants are witnessing swift regulatory approvals and growing physician acceptance globally.
Alk Positive Lung Cancer Treatment Market Insights, By Patient Demographics
Adult Patients dominate the market share, owing to the higher incidence of ALK-positive NSCLC predominantly in adults aged 40-60 years, representing the bulk of diagnosed cases and treatment volumes. The fastest growing demographic is the Geriatric Patients segment, reflecting improved diagnostic rates and tailored therapeutic regimens suitable for older populations with comorbidities. Pediatric Patients, though a minor segment, attract increasing research attention due to rare pediatric lung cancers with ALK aberrations, prompting specialized drug developments.
Alk Positive Lung Cancer Treatment Market Insights, By Distribution Channel
Hospital Pharmacy is dominating the market share due to direct oncology care delivery, availability of specialized drugs, and routine patient monitoring services, making it the primary source for ALK-positive lung cancer treatments. The fastest growing subsegment is Online Pharmacy, boosted by digital health adoption and increased patient preference for telemedicine-facilitated medicine purchases, especially post-pandemic. Retail Pharmacy serves as a traditional but essential access point, particularly in regions with well-established pharmaceutical retail networks.
Alk Positive Lung Cancer Treatment Market Trends
The Alk Positive Lung Cancer Treatment market is currently experiencing a transformation with the integration of combination therapies and precision diagnostic techniques, reshaping therapy protocols.
The adoption of AI-enabled diagnostics is enhancing early detection, exemplified by the U.S. FDA’s approval of several companion diagnostic tests in late 2024, contributing to more targeted treatment initiation.
Furthermore, expanding healthcare access in the Asia Pacific is fueled by government-backed initiatives aiming to boost cancer care infrastructure and affordability, exemplified by India’s National Cancer Control Program enhancements in 2025.
Market responsiveness to these trends underlines the critical interplay between technological innovation and policy support in shaping the Alk Positive Lung Cancer Treatment market trajectory.
Alk Positive Lung Cancer Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Alk Positive Lung Cancer Treatment Market Analysis and Trends
In North America, the dominance in the Alk Positive Lung Cancer Treatment market is fueled by advanced healthcare infrastructure, widespread reimbursement frameworks, and robust R&D ecosystems. The region accounts for over 38% of the market share, with the U.S. leading due to FDA approvals of novel ALK inhibitors and high patient diagnostic rates. Collaboration between pharmaceutical companies and healthcare providers further consolidates market revenue growth.
Asia Pacific Alk Positive Lung Cancer Treatment Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, with a CAGR surpassing 13% driven by increasing genetic testing penetration, expanding healthcare infrastructure, and rising patient awareness. Government initiatives and partnerships between global market players and regional vendors enable the rapid adoption of cutting-edge therapies, particularly in China and India.
Alk Positive Lung Cancer Treatment Market Outlook for Key Countries
USA Alk Positive Lung Cancer Treatment Market Analysis and Trends
The USA’s Alk Positive Lung Cancer Treatment market remains pivotal globally, accounting for the largest revenue share due to early diagnostic adoption and extensive insurance coverage. In 2024, over 30,000 new ALK-positive lung cancer diagnoses were reported, with the government supporting expanded access via Medicare and Medicaid reforms. Major companies such as Pfizer and Roche have introduced first- and second-generation ALK inhibitors, resulting in widespread therapy uptake and a strong pipeline of combination treatments under clinical evaluation, reinforcing the country’s strategic market position.
China Alk Positive Lung Cancer Treatment Market Analysis and Trends
China’s Alk Positive Lung Cancer Treatment market continues a rapid upward trajectory, underpinned by a substantial rise in diagnostic capabilities following national health policy reforms initiated in 2023. The surge in newly diagnosed patients—estimated to have increased by 22% in 2025 compared to 2023—has prompted intensified market activities from multinational companies, collaborating with local pharmaceutical entities. Government programs promoting genetic testing and oncology infrastructure enhancements have expanded patient access significantly, fostering strong business growth and an increasingly competitive landscape.
Analyst Opinion
Targeted therapy adoption drives market share expansion: The increasing prevalence of ALK gene rearrangements in approximately 5% of NSCLC patients globally is significantly boosting demand for ALK inhibitors. For instance, in 2024, over 50,000 new ALK-positive lung cancer cases were identified in North America alone, correlating directly with a 14% increase in therapy uptake compared to 2023. This demand-side trend strongly influences market revenue growth.
Pricing and reimbursement landscape evolves to support access: By mid-2025, key countries, including the U.S. and Germany, will have enhanced reimbursement policies for next-generation ALK inhibitors, making treatment more accessible. This has led to a 20% rise in patient treatment rates in Europe within one year, positively impacting market share within the Alk Positive Lung Cancer Treatment sector.
Expansion in emerging markets contributes significantly to market growth: Asia Pacific witnessed a notable surge in ALK-positive lung cancer diagnosis in 2025, with countries like China and India reporting double-digit growth in new patient numbers, partially due to increased genetic testing availability. This development supports the region’s fast-growing market revenue and industry size.
Pipeline and regulatory approvals accelerate market dynamics: The approval of third-generation ALK inhibitors by regulatory authorities in 2024 has diversified treatment approaches, providing clinicians with enhanced options for resistant cases. This advancement is anticipated to drive a compound increase of over 12% in market share by 2027, leading to substantial business growth opportunities in the Alk Positive Lung Cancer Treatment market.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 4.7 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.2% | 2032 Value Projection: |
USD 9.8 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Pfizer Inc., Novartis AG, Roche Holding AG, AstraZeneca plc, Bristol-Myers Squibb, Takeda Pharmaceutical Company Limited, Sanofi S.A., Eli Lilly and Company, Merck & Co., Inc., Johnson & Johnson, Bayer AG. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Alk Positive Lung Cancer Treatment Market Growth Factors
Several key factors are propelling growth in the Alk Positive Lung Cancer Treatment market. The rapid adoption of precision medicine technology, including widespread genetic testing capabilities, is enabling early and accurate diagnosis of ALK rearrangements. In 2024, diagnostic rates in developed economies improved by over 18%, driving treatment identification and consequent market scope expansion. Secondly, the introduction of next-generation ALK inhibitors with improved efficacy and reduced resistance profiles enhances clinical outcomes, resulting in increased patient adherence and extended treatment durations—critical elements in elevating overall market revenue. Thirdly, significant government initiatives advocating for improved cancer care infrastructure and reimbursement policies in regions like North America and Europe are spurring accelerated market growth. Finally, rising awareness campaigns and the growing patient advocacy movement contribute directly to early detection, aiding business growth and expanding industry size effectively.
Alk Positive Lung Cancer Treatment Market Development
In October 2025, Genprex, Inc. announced that its collaborators presented positive preclinical data for its lead gene-therapy candidate REQORSA® (quaratusugene ozeplasmid) at the AACR‑NCI‑EORTC International Conference on Molecular Targets and Cancer Therapeutics. The data focus on ALK-EML4-positive non-small cell lung cancer (NSCLC), demonstrating that REQORSA alone or in combination with an ALK inhibitor produced significant tumour shrinkage (~79 %) in a mouse model.
In June 2025, Nuvalent, Inc. announced major regulatory and clinical milestones for its targeted-oncology pipeline: a rolling NDA submission for its ROS1-selective inhibitor Zidesamtinib in TKI-pre-treated patients with advanced ROS1-positive NSCLC (accepted by the U.S. Food and Drug Administration under the Real-Time Oncology Review (RTOR) programme) and the launch of its global Phase 3 trial (ALKAZAR) for its ALK-selective inhibitor Neladalkib in TKI-naïve ALK-positive NSCLC.
Key Players
Leading Companies of the Market
Pfizer Inc.
Novartis AG
Roche Holding AG
AstraZeneca plc
Bristol-Myers Squibb
Takeda Pharmaceutical Company Limited
Sanofi S.A.
Eli Lilly and Company
Merck & Co., Inc.
Johnson & Johnson
Bayer AG
Among these, Novartis AG implemented a strategic global pricing adjustment in early 2025 for its second-generation ALK inhibitor, contributing to a 15% increase in its market penetration in Europe and North America. Pfizer accelerated R&D collaborations focusing on novel ALK inhibitors and combination regimens, leading to breakthrough approvals in 2024, which enhanced its competitive edge. Roche’s expansion into emerging markets through licensing agreements in the Asia Pacific augmented its geographic reach, improving its foothold amid rising patient bases.
Alk Positive Lung Cancer Treatment Market Future Outlook
The ALK-positive lung cancer treatment market is projected to expand steadily as molecular diagnostics become integrated into standard oncology protocols. Future growth will be shaped by next-generation inhibitors capable of overcoming resistance mutations, along with combination therapies targeting multiple signaling pathways. Advances in blood-based liquid biopsies will enable earlier detection and treatment personalization. Companies are increasingly focusing on optimizing drug tolerability and CNS activity to ensure comprehensive disease control. Continued investment in personalized oncology pipelines and regulatory approvals in emerging markets will further reinforce this segment as one of the fastest-evolving areas of targeted cancer therapy.
Alk Positive Lung Cancer Treatment Market Historical Analysis
The ALK-positive lung cancer treatment market emerged after the discovery of anaplastic lymphoma kinase (ALK) gene rearrangements in non-small cell lung cancer (NSCLC) in the late 2000s. The approval of the first ALK inhibitor, crizotinib, revolutionized treatment by introducing targeted therapy over traditional chemotherapy. Initially, resistance mutations and limited CNS penetration restricted long-term efficacy, but the development of second and third-generation ALK inhibitors such as ceritinib, alectinib, brigatinib, and lorlatinib addressed these challenges. Diagnostic advancements in next-generation sequencing and biomarker testing also improved detection rates, allowing precise patient targeting. Over time, ALK inhibitors became a cornerstone in precision oncology, significantly improving survival outcomes and quality of life for metastatic NSCLC patients.
Sources
Primary Research Interviews:
Oncologists
Molecular Pathologists
Pharmacologists
Clinical Research Coordinators
Databases:
NCI Cancer Research Data
PubChem Drug Data
WHO Global Health Observatory
Magazines:
Cancer Therapy Advisor
Oncology Times
PharmaVoice
BioPharma Dive
Journals:
Journal of Thoracic Oncology
The Lancet Oncology
Cancer Research
Nature Reviews Clinical Oncology
Newspapers:
The Washington Post (Health)
The Guardian (Science)
The Times of India (Health)
The Wall Street Journal (Pharma)
Associations:
American Society of Clinical Oncology (ASCO)
European Society for Medical Oncology (ESMO)
Lung Cancer Research Foundation
WHO
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients