Global Lung Cancer Market Size and Forecast – 2025 to 2032
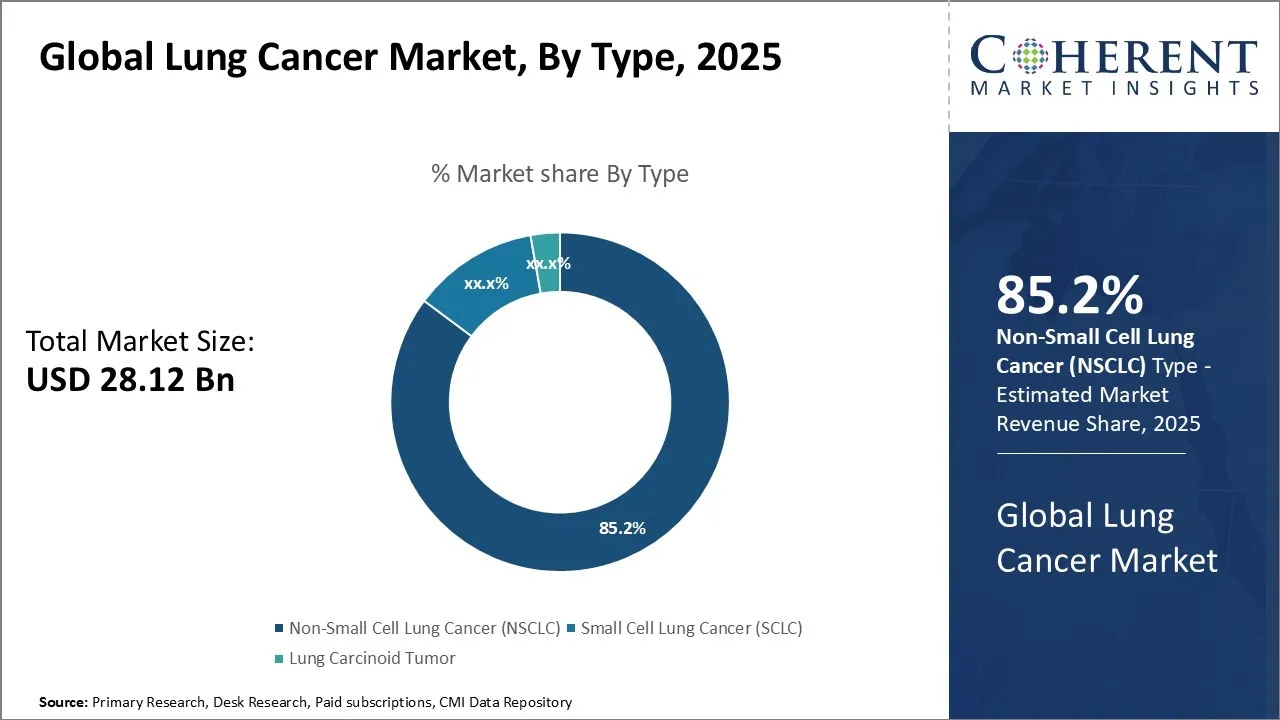
The Global Lung Cancer Market is estimated to be valued at USD 28.12 Bn in 2025 and is expected to reach USD 64.53 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 12.6% from 2025 to 2032. This significant growth is driven by increasing incidence rates, advancements in diagnostic technologies, and the development of targeted therapies, which are expanding the market footprint across regions.
Key Takeaways of the Global Lung Cancer Market
- Based on type, the Non-Small Cell Lung Cancer (NSCLC) segment is expected to hold the largest share of 85.2% in 2025 of the global lung cancer market.
- By drug class segment, cytotoxic chemotherapy continues to dominate with an estimated 35.8% market share in 2025.
- In terms of molecule type, biologics lead the market with an estimated 65.1% share in 2025.
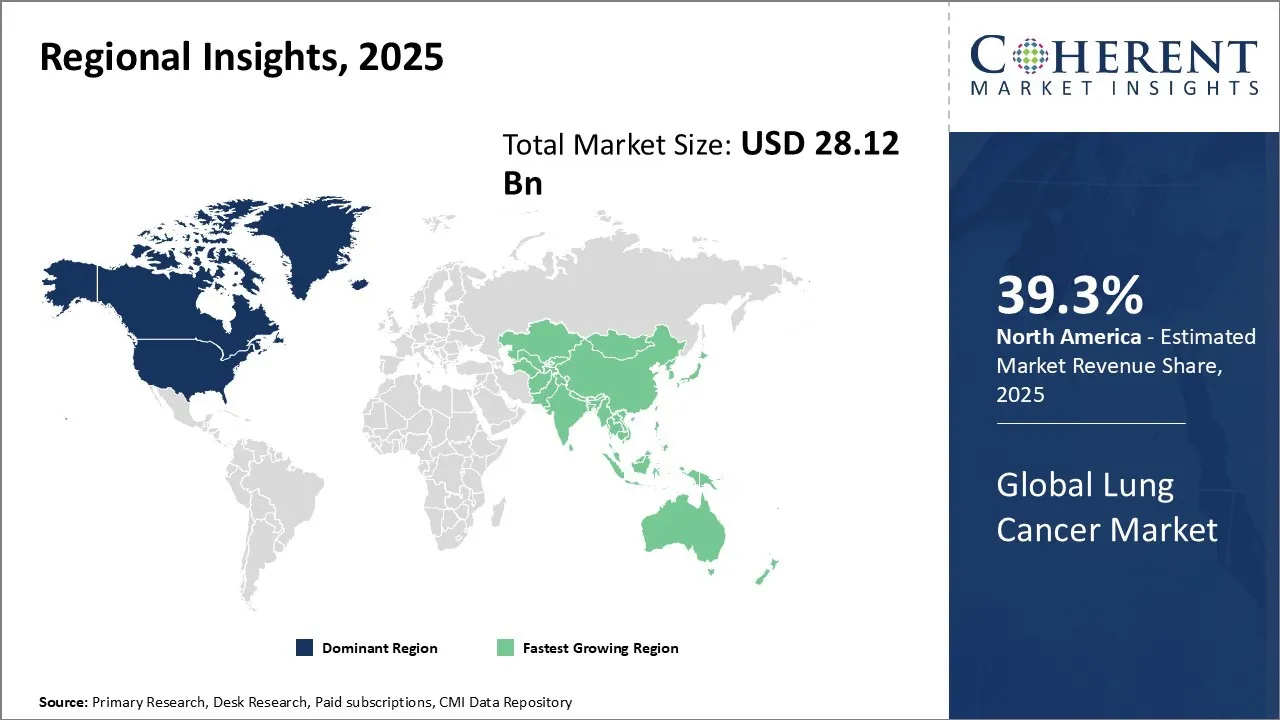
- North America is expected to lead the market, holding a share of 39.3% in 2025. Asia Pacific is anticipated to be the fastest-growing region, with a market share of 24.5% in 2025.
Market Overview
Market trends indicate a strong shift towards personalized medicine and immunotherapy in lung cancer treatment, with the increased adoption of biomarker testing enabling tailored therapeutic approaches. Additionally, growing investments in R&D and healthcare infrastructure, coupled with rising awareness and early detection initiatives, are accelerating the market expansion. The integration of artificial intelligence and digital health tools is further enhancing diagnostic accuracy and treatment efficacy, shaping the future landscape of the lung cancer market.
Currents Events and their Impact
|
Current Events |
Description and its impact |
|
GSK Receives U.S. FDA Breakthrough Therapy Designation for GSK5764227 in Small-Cell Lung Cancer |
|
|
Increased Focus on Immunotherapy as a Treatment Option |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Lung Cancer Market Insights, By Type - Dominance of Non-Small Cell Lung Cancer (NSCLC) is Driven by its High Prevalence and Diverse Treatment Landscape
Based on type, the Non-Small Cell Lung Cancer (NSCLC) segment is expected to command the highest share of 85.2% in 2025 primarily due to its predominant prevalence among lung cancer patients. This broad category of lung cancer types presents a wide patient population that mandates continued focus on therapeutic development and clinical management.
The high incidence of NSCLC relative to other lung cancer types fundamentally drives demand for more effective diagnostic and treatment options, making this segment the cornerstone of lung cancer therapeutics. Moreover, advances in molecular profiling and personalized medicine have significantly expanded targeted treatment options for NSCLC patients. The identification of specific genetic mutations such as EGFR, ALK, ROS1, and others has led to the development and adoption of precision therapies that improve clinical outcomes, further reinforcing NSCLC’s market dominance.
Lung Cancer Market Insights, By Drug Class - Cytotoxic Chemotherapy’s Leading Position is Sustained by its Broad Application and Accessibility
Within the drug class segment, cytotoxic chemotherapy is expected to maintain the dominant market share of 35.8% in 2025 due to its long-standing role as a foundational treatment modality for lung cancer. Cytotoxic agents, including alkylating agents like cisplatin and carboplatin, antimetabolites like pemetrexed and gemcitabine, mitotic inhibitors such as paclitaxel and docetaxel, and topoisomerase inhibitors like etoposide, have well-established efficacy profiles in treating various lung cancer types.
One of the primary drivers of cytotoxic chemotherapy’s extensive application is its broad utility in both small cell and non-small cell lung cancers. It often serves as either the first-line treatment or as part of combination regimens in advanced-stage disease where targeted options may be limited due to lack of actionable mutations.
In August 2024, Johnson & Johnson, a global healthcare leader, announced that the U.S. FDA had approved RYBREVANT (amivantamab-vmjw) in combination with LAZCLUZE (lazertinib) for the first-line treatment of EGFR-mutated advanced non-small cell lung cancer. This chemotherapy-free regimen, the first of its kind, demonstrated superior progression-free survival compared to osimertinib in the Phase 3 MARIPOSA trial, reducing the risk of disease progression or death by 30% with a median duration of response nine months longer.
Lung Cancer Market Insights, By Molecule Type – Biologics Dominates Through Immunotherapy Advancements and Superior Survival Outcomes
By molecule type segmentation, biologics have emerged as the dominant segment in the global lung cancer market with an estimated 65.1% in 2025, largely due to the transformative role of immunotherapies and targeted antibody-based treatments. Biologics, including PD-1/PD-L1 inhibitors such as pembrolizumab and atezolizumab, as well as antibody-drug conjugates like datopotamab deruxtecan, have redefined treatment outcomes for patients across multiple lines of therapy. Their mechanism of harnessing the immune system to selectively target and destroy cancer cells has delivered superior survival benefits, particularly in advanced and metastatic non-small cell lung cancer (NSCLC).
The dominance of biologics is also supported by their ability to address high unmet needs in patients resistant to chemotherapy or small molecule therapies. With advancements in companion diagnostics, patient selection for biologic-based treatments has become more precise, ensuring better efficacy and fewer off-target effects. Additionally, the continuous innovation in bispecific antibodies, immune checkpoint inhibitors, and next-generation antibody-drug conjugates is expanding their scope beyond NSCLC to include small cell lung cancer (SCLC) as well.
Reimbursement Scenario
- According to American Lung Association, lung cancer screening reimbursement has evolved significantly to encourage early detection and improve survival rates. Coverage varies by payer type, but federal and state programs, including Medicare and Medicaid, play a key role in making screening accessible. For example, Medicare provides coverage for lung cancer screening with low-dose computed tomography (LDCT) for individuals aged 50-77 years with a history of smoking, and waives coinsurance and deductibles for this preventive service. Medicaid coverage differs by state, with 49 states offering coverage under their fee-for-service programs, though coverage details may vary across Medicaid managed care plans.
- Private insurance also generally follows the guidelines set by the Affordable Care Act (ACA), requiring coverage for USPSTF recommended services like LDCT screenings at no cost to the member. However, some employer-sponsored plans may not fully cover the screening if they are “grandfathered” under ACA rules, potentially leading to cost-sharing. Similarly, some individual health plans and non-ACA compliant insurance plans may not cover the screening or impose additional costs. Understanding these billing and reimbursement nuances is essential for healthcare providers to ensure that eligible patients can access lung cancer screening without financial barriers.
Regional Insights
To learn more about this report, Download Free Sample
North America Lung Cancer Market Analysis and Trends
North America’s dominance in the global lung cancer market, with an estimated share of 39.3% in 2025, is driven by a highly advanced healthcare infrastructure, extensive research and development activities, and strong government support for cancer awareness and treatment programs. The U.S. and Canada possess a robust ecosystem of pharmaceutical companies, biotechnology firms, and healthcare providers that focus on innovative lung cancer therapies, including targeted therapies and immunotherapies. Supportive regulatory frameworks, such as expedited drug approvals by the USFDA, and substantial public and private investments in oncology research, further reinforce the region's leading position. Notable companies like Pfizer, Bristol-Myers Squibb, and AstraZeneca have significant operations centered in North America, contributing novel lung cancer drugs and diagnostic tools.
In August 2022, Amgen, a U.S.-based biotechnology leader, and AstraZeneca, a global pharmaceutical major, started a Phase 3 clinical trial in extensive-stage small-cell lung cancer. The study will test whether adding Amgen’s experimental therapy Tarlatamab to AstraZeneca’s immunotherapy Durvalumab plus standard chemotherapy (carboplatin and etoposide) can improve survival outcomes in first-line patients.
Asia Pacific Lung Cancer Market Analysis and Trends
The Asia Pacific region is expected to exhibit the fastest growth in the global lung cancer market with a share of 24.5% in 2025, fueled by rising healthcare expenditures, growing awareness, and increasing prevalence of lung cancer, especially due to urbanization and higher smoking rates in countries like China and India. Governments across the region are rapidly enhancing healthcare infrastructure and implementing favorable policies to improve cancer care accessibility and affordability.
Emerging markets like China, Japan, South Korea, and India are witnessing increased participation from both global pharmaceutical players and thriving domestic companies such as Takeda, Sun Pharmaceutical, and WuXi AppTec. Trade liberalization and strategic partnerships between local firms and multinational corporations have facilitated knowledge transfer, improved drug availability, and accelerated clinical research efforts. Additionally, growing investments in biotechnology and diagnostics are fostering innovation that caters specifically to the genetic profiles and treatment needs of the local populations.
Global Lung Cancer Market Outlook for Key Countries
U.S. Lung Cancer Market Trends
The U.S. lung cancer market is characterized by a mature and competitive landscape featuring major global pharmaceutical giants like Merck, Roche, and Novartis. These companies have pioneered key immunotherapies and targeted drugs that have transformed lung cancer treatment protocols. The country’s well-developed health insurance systems and widespread access to advanced diagnostics contribute to early detection and personalized treatment approaches. Collaborations between government agencies such as the National Cancer Institute and private sector players further drive innovation and clinical trial activities, strengthening the U.S. position as a global frontrunner.
In May 2025, AbbVie, a U.S.-based global biopharmaceutical company, received the U.S. FDA accelerated approval for EMRELIS (telisotuzumab vedotin-tllv) to treat adults with advanced non-squamous non-small cell lung cancer (NSCLC) who have high c-Met protein overexpression and have received prior therapy. EMRELIS, a c-Met-directed antibody-drug conjugate, is the first and only approved treatment for this patient group, offering a targeted option for a population with poor prognosis and limited choices.
China Lung Cancer Market Trends
China’s lung cancer market has rapidly expanded due to increased government focus on cancer control programs and healthcare modernization. Companies like BeiGene and Shanghai Fosun are playing critical roles by developing novel therapies and pushing for domestic innovation alongside multinational firms like AstraZeneca and Pfizer. Large-scale screening programs and growing healthcare coverage have improved early diagnosis rates. Additionally, regulatory reforms aimed at faster drug approvals and increased investment in medical infrastructure have enhanced patient access to cutting-edge treatments, fueling market growth.
In June 2025, HUTCHMED, a Hong Kong-based biopharmaceutical company, received approval in China for the combination of ORPATHYS (savolitinib) and TAGRISSO (osimertinib), developed with AstraZeneca, to treat advanced non-small cell lung cancer patients with MET amplification after progression on EGFR inhibitor therapy.
Germany Lung Cancer Market Trends
Germany’s lung cancer market is distinguished by a strategic healthcare ecosystem that combines comprehensive cancer care centers with advanced research institutions. Leading companies like Bayer and Boehringer Ingelheim contribute through innovative drug portfolios and active participation in global clinical trials. Germany’s strong regulatory environment and government initiatives focused on cancer prevention, early detection, and treatment enable effective integration of new therapies into standard care.
In November 2024, University Hospital Cologne, one of Germany’s leading cancer research centers, and Gustave Roussy, a top French comprehensive cancer institute, launched REDUCE-LUNG, a USD 1.7 million Franco-German study focused on improving treatment outcomes for lung cancer patients over 70. The three-year trial will test whether reduced-intensity chemo-immunotherapy followed by maintenance can be as effective but better tolerated than standard treatment.
India Lung Cancer Market Trends
India’s lung cancer market is expanding as a result of increased healthcare investments, rising disease awareness, and growing availability of affordable diagnostic and treatment options. Indian pharmaceutical firms like Cipla and Dr. Reddy’s Laboratories engage actively in producing generic lung cancer medications and biosimilars, making treatments more accessible to a broader population. The government’s push for better healthcare infrastructure, along with strategic collaborations with multinational corporations, has enhanced clinical research and treatment capabilities.
In June 2025, Glenmark Pharmaceuticals, a leading Indian drugmaker, launched the lung cancer treatment drug Tevimbra in India after approval from the Central Drugs Standard Control Organisation. Developed by BeiGene (now BeOne Medicines), a global oncology leader, Tevimbra’s entry marks Glenmark’s first step into immune-oncology in the country and a significant milestone in building its innovative oncology portfolio.
End User Feedback and Unmet Needs in the Global Lung Cancer Market
- End users in the global lung cancer market, including healthcare providers, patients, and research institutions, have consistently highlighted both strengths and areas for improvement in available treatments and diagnostic technologies. A positive example of user satisfaction comes from healthcare providers who have praised the efficacy of advanced targeted therapies, which have shown significant promise in improving patient outcomes. Specifically, treatments like immune checkpoint inhibitors have garnered positive feedback for their ability to extend survival rates in advanced stages of lung cancer, offering a higher quality of life for patients. However, concerns remain regarding the accessibility and cost of these treatments, which have been widely cited as barriers by both providers and patients, particularly in low- and middle-income countries. Additionally, a lack of integration between diagnostic tools and treatment plans has often led to delays in care, with some institutions reporting difficulties in aligning the latest genomic testing results with personalized treatment options.
- Despite advances in the field, unmet needs persist, particularly in terms of affordability, technological gaps, and customization. Many end-users have voiced concerns over the high cost of cutting-edge treatments, which remain prohibitive for a significant portion of the patient population. Furthermore, there is a notable demand for more personalized solutions, with healthcare providers expressing the need for more advanced technologies that can better integrate data from diverse diagnostic tools, such as imaging, genetic testing, and patient history. By addressing these gaps, manufacturers and developers could open new avenues for growth, enhancing customer retention through more tailored and cost-effective solutions. Innovation in accessible, customizable, and integrated treatment models could drive market expansion and improve overall patient care.
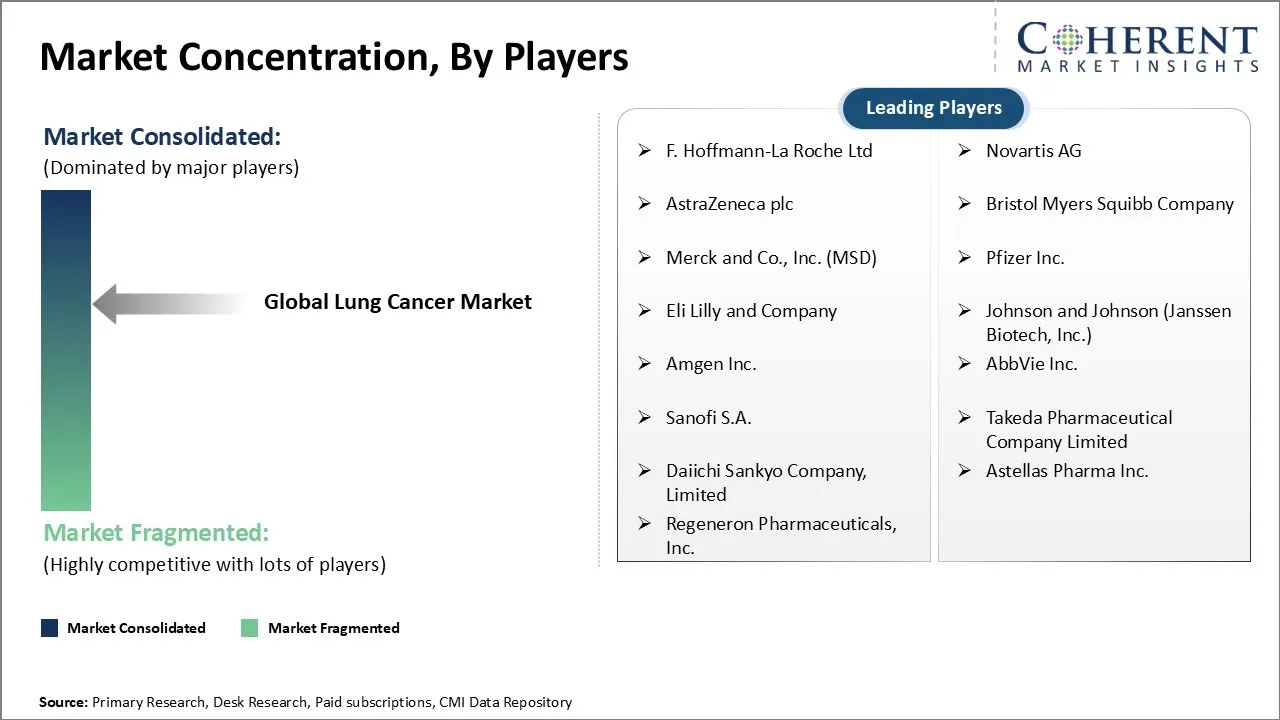
Market Players, Key Developments, and Competitive Intelligence
To learn more about this report, Download Free Sample
Key Developments
- On August 18, 2025, Daiichi Sankyo, a Japan-based global pharma company, and Merck & Co., Inc. (known as MSD outside the US and Canada), received the U.S. FDA Breakthrough Therapy Designation for ifinatamab deruxtecan (I-DXd), a first-in-class B7-H3 directed antibody-drug conjugate, for patients with extensive-stage small cell lung cancer who progressed after platinum chemotherapy.
- On August 5, 2025, Intas Pharmaceuticals, one of India’s leading pharma companies, launched HETRONIFLY (Serplulimab), the country’s first novel immunotherapy for advanced Small Cell Lung Cancer. The drug, developed through a licensing agreement with Shanghai Henlius Biotech, a global biotech player, is the world’s first PD-1 inhibitor approved for Extensive-Stage Small Cell Lung Cancer and has already been introduced in Europe and over 40 countries.
- In June 2025, Amgen Inc., a leading U.S. biotechnology company, announced Phase 3 trial results showing its drug IMDELLTRA (tarlatamab-dlle) cut the risk of death by 40% and extended survival by over five months compared to chemotherapy in small cell lung cancer patients who had progressed after platinum-based treatment.
- In June 2025, Datroway (Datopotamab Deruxtecan, Dato-DXd) received the U.S. Food and Drug Administration (FDA) accelerated approval as the first Trophoblast Cell-Surface Antigen 2 (TROP2)-directed therapy for advanced Epidermal Growth Factor Receptor (EGFR)-mutated Non-Small Cell Lung Cancer (NSCLC) after prior treatments. AstraZeneca, a global oncology leader, and Daiichi Sankyo, its Japanese biopharmaceutical partner, co-developed the drug, while LUNGevity Foundation welcomed the approval as a crucial new option for patients with limited choices.
Top Strategies Followed by Global Lung Cancer Market Players
- Established companies dominate the landscape by heavily investing in research and development (R&D) to innovate high-performance products that address unmet clinical needs. These market leaders leverage their substantial financial resources to accelerate the development of advanced diagnostic tools, targeted therapies, and immunotherapies to maintain a competitive edge. In addition to innovation, forming strategic partnerships with other major industry players and original equipment manufacturers (OEMs) is a cornerstone of their approach.
- In May 2024, Pfizer, a global biopharmaceutical company, reported landmark results from its Phase 3 CROWN trial, showing that 60% of patients with ALK-positive advanced non-small cell lung cancer treated with LORBRENA (lorlatinib) were alive without disease progression after five years, compared to just 8% on XALKORI (crizotinib).
- Mid-level players in the lung cancer market adopt a different set of strategies tailored to their resource capabilities and market positioning. Their primary focus lies in offering cost-effective solutions that strike a balance between quality and affordability, targeting the price-sensitive segments of both developed and emerging markets. By doing so, they cater to a broader patient population that may otherwise remain underserved due to high treatment costs.
- In March 2025, Alkem Laboratories, a leading Indian pharmaceutical company, launched its affordable generic version of empagliflozin in India under the brand name Empanorm, priced nearly 80% lower than the innovator products. Used to manage type-2 diabetes, chronic kidney disease, and heart failure, Empanorm and its combination variants (Empanorm L, Empanorm Duo/Alsita E, and Empanorm M) are bioequivalent to innovator brands.
- Small-scale players in the global lung cancer market typically differentiate themselves by focusing on niche segments and specialized features that larger competitors may overlook. These companies often invest in cutting-edge technologies such as AI-driven diagnostics, liquid biopsies, or personalized medicine platforms to develop innovative products that offer distinct advantages. By concentrating on innovation and agility, they stay competitive despite limited resources and market reach.
- In October 2024, Novocure, a global oncology company known for its Tumor Treating Fields (TTFields) therapy, received the U.S. FDA approval for its device Optune Lua to be used alongside PD-1/PD-L1 inhibitors or docetaxel in adults with metastatic non-small cell lung cancer who progressed after platinum-based chemotherapy.
Market Report Scope
Lung Cancer Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 28.12 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.6% | 2032 Value Projection: | USD 64.53 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
F. Hoffmann-La Roche Ltd, Novartis AG, AstraZeneca plc, Bristol Myers Squibb Company, Merck and Co., Inc. (MSD), Pfizer Inc., Eli Lilly and Company, Johnson and Johnson (Janssen Biotech, Inc.), Amgen Inc., AbbVie Inc., Sanofi S.A., Takeda Pharmaceutical Company Limited, Daiichi Sankyo Company, Limited, Astellas Pharma Inc., and Regeneron Pharmaceuticals, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
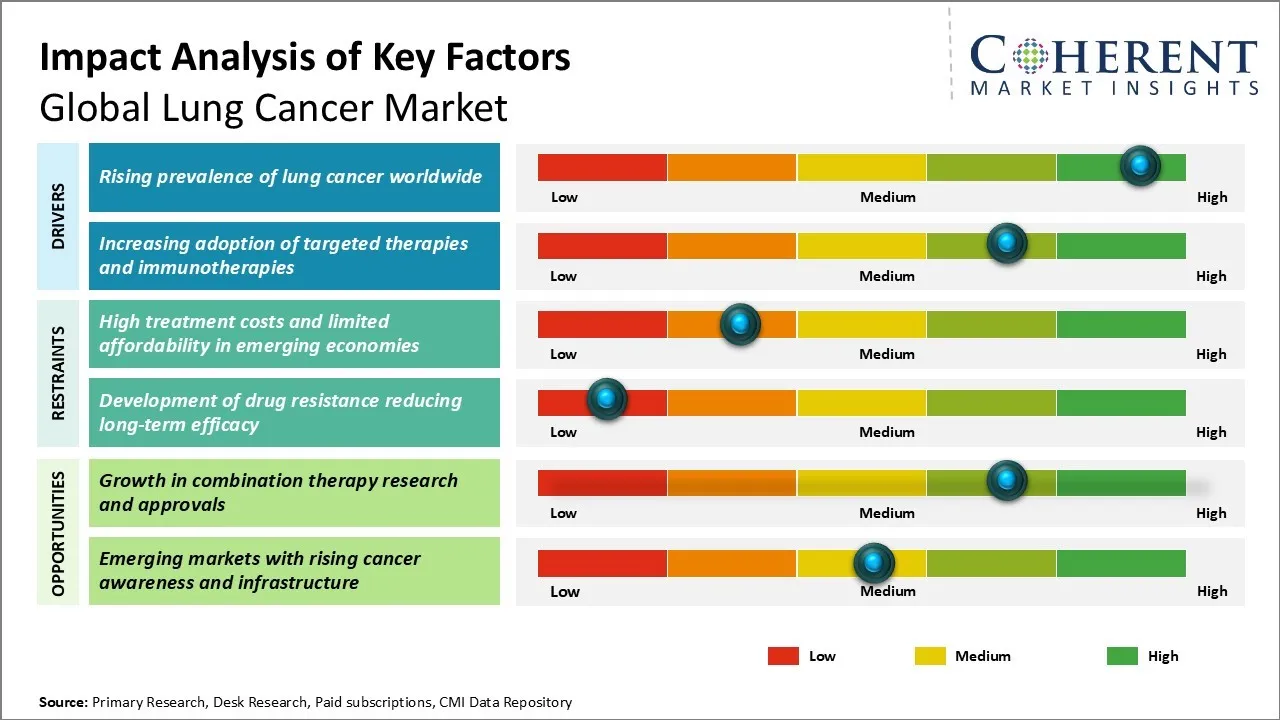
Lung Cancer Market Dynamics
To learn more about this report, Download Free Sample
Lung Cancer Market Driver - Rising Prevalence of Lung Cancer Worldwide
The increasing prevalence of lung cancer globally is a significant driver influencing the dynamics of the lung cancer market. This rise can be attributed to several factors, including widespread tobacco consumption, exposure to environmental pollutants, and occupational hazards, all of which contribute to higher incidence rates across various regions. Additionally, aging populations in many countries are more susceptible to developing lung cancer due to cumulative exposure to carcinogens over time and weakened immune responses. Advances in diagnostic technologies have also led to improved detection rates, identifying more cases that might previously have gone undiagnosed. Moreover, urbanization and industrialization in developing nations have exacerbated exposure to air pollution, further fueling the risk of lung cancer. The burden of lung cancer, therefore, spans across both developed and developing economies, underscoring an urgent need for better screening, early diagnosis, and treatment options.
In February 2024, the World Health Organization (WHO) and its cancer agency IARC reported that lung cancer remains the most common and deadliest cancer worldwide, with 2.5 million new cases and 1.8 million deaths in 2022. The rising prevalence is closely linked to persistent tobacco use in Asia and growing exposure to air pollution. Despite advances in treatment, inequities remain stark, as access to lung cancer care is four to seven times higher in high-income countries than in lower-income ones. With global cancer cases projected to rise by 77% by 2050, lung cancer continues to drive a major share of the burden, highlighting urgent gaps in prevention, early detection, and treatment access.
Lung Cancer Market Opportunity - Growth in Combination Therapy Research and Approvals
The global lung cancer market is witnessing a significant opportunity driven by the rapid advancement and increasing approvals of combination therapies. Combination therapy, which involves using two or more treatment modalities such as targeted therapy, immunotherapy, chemotherapy, or radiation in tandem, is gaining traction due to its potential to improve patient outcomes by attacking cancer through multiple mechanisms simultaneously. Recent clinical trials have demonstrated that these therapies can enhance efficacy, delay disease progression, and overcome resistance often seen with monotherapies. Regulatory bodies, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have expedited approvals for numerous combination regimens, reflecting their clinical benefit and growing acceptance.
In June 2025, Jazz Pharmaceuticals, a Dublin-based biopharma company, announced that its drug Zepzelca (lurbinectedin) combined with atezolizumab (Tecentriq) from Roche, a global oncology leader, significantly improved survival in patients with extensive-stage small cell lung cancer in the Phase 3 IMforte study.
Analyst Opinion (Expert Opinion)
- The lung cancer market is evolving rapidly, supported by breakthroughs in targeted therapies and immunotherapies, alongside greater use of biomarker testing and precision medicine. Increasing awareness programs, regulatory backing for accelerated drug approvals, and rising investments in oncology R&D continue to shape the market’s growth trajectory. At the same time, challenges such as high treatment costs, disparities in access across regions, and the emergence of drug resistance remain significant hurdles. Opportunities are also expanding as novel drug classes like KRAS inhibitors and antibody–drug conjugates gain momentum, while clinical collaborations and digital health integration open fresh avenues for patient care. Conferences such as the IASLC World Conference on Lung Cancer, ASCO Annual Meeting, and ESMO Congress in the last few years have been central in bringing together researchers, clinicians, and policymakers, offering platforms that drive scientific exchange, inform regulatory guidelines, and highlight next-generation treatment approaches.
- Several real-world initiatives underline this progress. For example, AstraZeneca’s expansion of Tagrisso into adjuvant therapy and Bristol Myers Squibb’s approvals for dual immunotherapy combinations have significantly widened treatment choices. Governments in Europe and Asia have strengthened early screening and reimbursement frameworks, while public–private partnerships are investing in lung cancer awareness campaigns and clinical trial networks. These developments not only accelerate patient access to innovative treatments but also foster long-term sustainability in lung cancer management, making the market more dynamic and competitive in the years ahead.
Market Segmentation
- Type Insights (Revenue, USD Bn, 2020 - 2032)
- Non-Small Cell Lung Cancer (NSCLC)
- Adenocarcinoma
- Squamous Cell Carcinoma
- Large Cell Carcinoma
- Small Cell Lung Cancer (SCLC)
- Lung Carcinoid Tumor
- Non-Small Cell Lung Cancer (NSCLC)
- Drug Class Insights (Revenue, USD Bn, 2020 - 2032)
- Cytotoxic Chemotherapy
- Alkylating agents (cisplatin, carboplatin, etc.)
- Antimetabolites (pemetrexed, gemcitabine)
- Mitotic inhibitors (taxanes: paclitaxel, docetaxel)
- Topoisomerase inhibitors (etoposide, irinotecan)
- Others
- Targeted Therapy
- EGFR Inhibitors (Erlotinib, etc.)
- ALK Inhibitors (Crizotinib, etc.)
- ROS1 Inhibitors (Entrectinib, etc.)
- BRAF/MEK Inhibitors (Dabrafenib, etc.)
- MET Inhibitors (Capmatinib, etc.)
- RET Inhibitors (Selpercatinib, etc.)
- HER2 Inhibitors (Trastuzumab, etc.)
- Immunotherapy
- PD-1 Inhibitors (Nivolumab, etc.)
- PD-L1 Inhibitors (Atezolizumab, etc.)
- CTLA-4 Inhibitors (Ipilimumab, etc.)
- Other Combination Therapeutics
- Cytotoxic Chemotherapy
- Molecule Type Insights (Revenue, USD Bn, 2020 - 2032)
- Small Molecules
- Biologics
- Route of Administration Insights (Revenue, USD Bn, 2020 - 2032)
- Parenteral
- Oral
- Stage of Disease Insights (Revenue, USD Bn, 2020 - 2032)
- Early-stage (Stage I–II)
- Locally Advanced (Stage III)
- Metastatic/Advanced (Stage IV)
- Age Group Insights (Revenue, USD Bn, 2020 - 2032)
- Adult
- Pediatric
- Geriatric
- Gender Insights (Revenue, USD Bn, 2020 - 2032)
- Male
- Female
- Distribution Channel Insights (Revenue, USD Bn, 2020 - 2032)
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- End User Insights (Revenue, USD Bn, 2020 - 2032)
- Hospitals
- Diagnostic Laboratories
- Specialty Cancer Clinics
- Homecare Settings
- Others (Research and Academic Institutes, etc.)
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Hoffmann-La Roche Ltd.
- Novartis AG
- AstraZeneca plc
- Bristol Myers Squibb Company
- Merck and Co., Inc. (MSD)
- Pfizer Inc.
- Eli Lilly and Company
- Johnson and Johnson (Janssen Biotech, Inc.)
- Amgen Inc.
- AbbVie Inc.
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
- Daiichi Sankyo Company, Limited
- Astellas Pharma Inc.
- Regeneron Pharmaceuticals, Inc.
Sources
Primary Research Interviews
- Industry Stakeholders list
- Oncologists specializing in thoracic cancers
- Pharmaceutical R&D executives
- End Users list
- Hospital oncology departments
- Cancer patient advocacy groups
Government and International Databases
- World Health Organization (WHO)
- International Agency for Research on Cancer (IARC)
- Centers for Disease Control and Prevention (CDC)
- National Cancer Institute (NCI)
- European Medicines Agency (EMA)
- U.S. Food and Drug Administration (FDA)
Trade Publications
- PharmaTimes
- FiercePharma
- BioPharma Dive
- Cancer Therapy Advisor
- The Pharma Letter
- Oncology Times
Academic Journals
- The Lancet Oncology
- Journal of Clinical Oncology (JCO)
- Cancer Research
- Nature Reviews Cancer
- Lung Cancer Journal (Elsevier)
- JAMA Oncology
Reputable Newspapers
- The New York Times (Health Section)
- The Guardian (Health/Science)
- Financial Times (Healthcare/Pharma coverage)
- The Washington Post (Health)
- The Times of India (Health/Science)
- The Wall Street Journal (Pharma & Health)
Industry Associations
- American Society of Clinical Oncology (ASCO)
- European Society for Medical Oncology (ESMO)
- International Association for the Study of Lung Cancer (IASLC)
- American Cancer Society (ACS)
- Lung Cancer Research Foundation (LCRF)
- National Comprehensive Cancer Network (NCCN)
Public Domain Resources
- SEER Database (Surveillance, Epidemiology, and End Results)
- OECD Health Statistics
- World Bank Health Data
- Eurostat
Proprietary Elements
- CMI Data Analytics Tool: Proprietary analytics tool to analyze real-time market trends, consumer behavior, and technology adoption in market
- Proprietary CMI Existing Repository of Information for Last 8 Years
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients