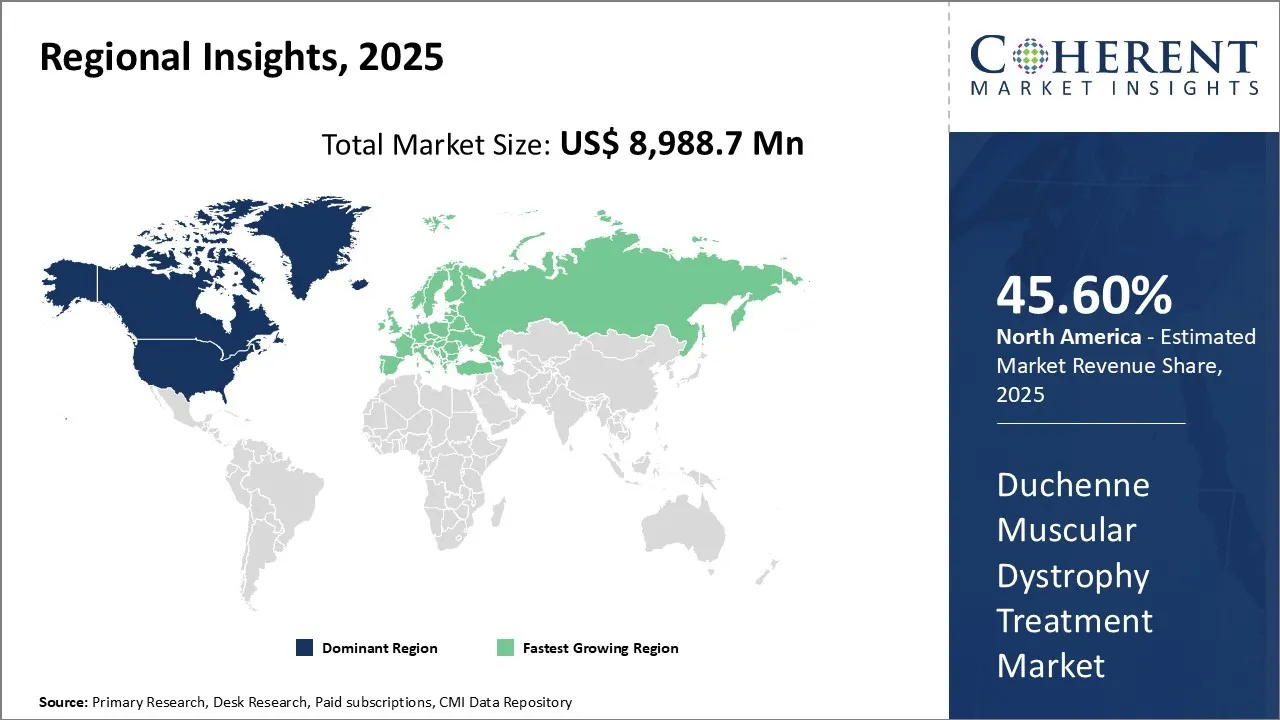
The duchenne muscular dystrophy treatment market size is estimated to be valued at US$ 8,988.7 Mn in 2025 and is expected to reach US$ 138,818.1 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 47.85% from 2025 to 2032.
The increasing awareness of rare genetic disorders and heightened emphasis on cutting-edge therapeutic options are driving the duchenne muscular dystrophy treatment market growth. Fueled by continued research in gene therapy, exon skipping methods, and corticosteroid innovations, the treatment space is witnessing exponential growth. Rising R&D spending combined with favorable regulatory environments and orphan drug designations are increasing the availability and efficacy of DMD treatments. Besides, the increasing pool of patients and early diagnosis due to genetic testing are favoring the utilization of targeted therapies. However, high cost for duchenne muscular dystrophy treatment and limited availability of curative options continue to pose challenges to the duchenne muscular dystrophy treatment market growth.
|
Current Events |
Description and its impact |
|
Regulatory Changes and Healthcare Policy Reforms
|
|
|
Public Health Trends
|
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The duchenne muscular dystrophy treatment market is undergoing a major shift with the increasing adoption of digital biomarkers and wearable technologies in clinical trials. These technologies are increasingly being utilized to track motor function and disease progression in real time, improving the accuracy and consistency of trial results. Technologies like smartwatches, motion detection devices, and artificial intelligence-based gait analysis tools deliver ongoing data collection capabilities, facilitating a better insight into patient mobility than through sporadic clinic appointments.
For example, in August 2023, the European Medicines Agency (EMA) licensed Stride Velocity 95th Centile (SV95C) as an acceptable clinical trial outcome measure for DMD. This metric, the speed of the top 5% of a patient's strides, is recorded with the ActiMyo wearable device. The device features wrist or ankle sensors and provides more natural, consistent monitoring of mobility than traditional metrics such as the 6-minute walk test. This regulatory clearance represents a move toward real-world, technology-based clinical assessment techniques that are less invasive and patient-centric.
Duchenne muscular dystrophy treatment market is witnessing an increasing wave of strategic partnerships, licensing agreements, and mergers & acquisitions (M&A), particularly between large pharmaceutical companies and biotech firms focused on genetic and RNA-based therapies. These collaborations are aimed at pooling resources, accelerating research and development, and gaining a competitive edge in a rapidly evolving therapeutic area.
For instance, in January 2022, Capricor Therapeutics, a biotechnology company focused on the development of transformative cell and exosome-based therapeutics for the treatment and prevention of a broad spectrum of diseases, announced that it had entered into a partnership with Nippon Shinyaku Co., Ltd., a Japan-based pharmaceutical company listed on the TYO, for the exclusive commercialization and distribution in the U.S. of Capricor’s lead asset, CAP-1002, for the treatment of Duchenne muscular dystrophy (DMD), a rare neuromuscular disease with limited treatment options.
Lack of standardization to measure clinical efficacy across all stages of DMD is one of the vital factors expected to hamper growth of the global duchenne muscular dystrophy treatment market. There are quite a few on-going clinical trials in DMD, which use the diverse outcome measures to quantify the disease progression, including the 6-minutewalking test, the performance of the upper limb, the time taken to rise from the floor, the time taken to climb four steps, and the north star ambulatory assessment scale. There is need for other better outcome measures which may capture the progression between ambulant and non-ambulant phases of the disease.
Another factor which is hampering growth of the global duchenne muscular dystrophy treatment market is the stringent rules and regulations. Regulatory agencies like the U.S. FDA and the European Medicines Agency have strict rules and procedures for approving new drugs and therapies. Thus, very few companies (pharmaceutical companies spend huge amounts on the R&D of drugs) come into front for the new drug research for the rare diseases because it needs bigger funds.
The increasing development of gene therapy and targeted medicine offers a vast opportunity for expansion of the duchenne muscular dystrophy treatment market. With increasing investments in precision medicine and biotechnology, there is greater potential to create drugs that address the underlying cause of DMD at the genetic level, with long-term or even curative effects.
Additionally, continuous research, combined with pharma and research institution alliances, is pushing the discovery of new therapeutic candidates faster. Increased emphasis on patient registries, real-world data, and biomarker identification further supports the creation of individualized treatment regimens. With changing science and technology, these technologies are likely to unlock new business opportunities and tremendously increase the market for DMD treatments in the future years.
Based on therapeutic approach & treatment type, the market is segmented into molecular-based therapies (mutation suppression and exon skipping), steroid therapy (corticosteroids), nonsteroidal anti-inflammatory drugs (NSAIDs), and other therapeutic approaches & treatment types. Molecular-based therapies segment is further segmented into mutation suppression and exon skipping. Out of which, exon skipping segment is expected to dominate the market because it has the highest potential to actually change the trajectory of duchenne muscular dystrophy (DMD) at the level of genes.
Exon skipping therapies are designed to skip faulty exons within the dystrophin gene so that a working dystrophin protein can be produced, important for muscle function. These treatments should pick up considerable momentum in the market since they can correct the genetic root cause of DMD, having more targeted and possibly longer-term effects than existing treatments.
For instance, in February 2023, SQY Therapeutics, sponsor of the "Avance1" clinical trial, is pleased to announce that patient enrolment has begun, following the authorization for clinical trial by the ANSM (The French National Agency for the Safety of Medicines) in the framework of the European regulation on clinical trials of medicinal products, number EU CT 2022-500703-49-01.
By end user, the hospitals/clinics segment is expected to dominate with the highest market share of 56.4% in 2025 due to the growing incidence of duchenne muscular dystrophy (DMD) and a demand for tertiary care offered at hospital and clinic facilities. Hospitals and clinics provide a variety of services, including monitoring, diagnostics, and sophisticated therapeutic interventions that are critical to the treatment of DMD. For instance, in March 2024, Sheikh Khalifa Medical City (SKMC) in Abu Dhabi administered delandistrogene moxeparvovec (Elevidys), a gene therapy for DMD, marking the first time this treatment was provided outside the United States. This milestone was achieved through collaboration between SKMC and the Department of Health – Abu Dhabi, highlighting the capability of hospital settings to deliver cutting-edge treatments.
To learn more about this report, Download Free Sample
Among regions, North America is expected to gain highest share in the market over the forecast period owing to the rising disease burden of Duchenne muscular dystrophy, introduction of novel therapies, high healthcare expenditure, and rise in awareness among people in the region.
For instance, market players operating in the industry are focusing on launching novel drugs or therapies for the treatment of DMD. In May 2022, Elamipretide received Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) Office of Orphan Products Development for the treatment of patients with Duchenne muscular dystrophy.
Europe is a promising growth region for the Duchenne muscular dystrophy treatment market, supported by encouraging regulatory policies, improving disease awareness, and a rising demand for orphan drugs. DMD affects around one in 3 500 new-born boys. It is therefore classed as a ‘rare’ disease – despite the fact that the European Medicines Agency (EMA) estimates that approximately 26 000 people suffer from the condition in Europe. Moreover, in December 2023, vamorolone (Agamree) was approved by the European Commission for the age group of four years and above, in tune with Europe's positive approach to rare disease treatment.
The Asia Pacific region is likely to experience the most rapid expansion in the market for treating DMD as a consequence of increasing healthcare spending, enhanced access to diagnostics, and increasing numbers of undiagnosed patients. Japan, China, and India are making investments in rare disease publicity campaigns as well as increasing research partnerships with Western drug firms. Japan has been a significant location for clinical trials involving exon-skipping drugs. The region's growing focus on early genetic screening and the addition of DMD to newborn screening programs are expected to improve diagnosis and treatment access in the next few years.
The U.S. remains the hub of DMD treatment innovation, powered by robust biotech ecosystems, early launches, and favorable policies. As of 2023, the U.S. had more than 16,000 prevalent cases of DMD, generating strong demand for novel treatments. Top-tier institutions like Nationwide Children's Hospital have been pioneers in adopting gene therapies such as Elevidys. Also, programs under the Orphan Drug Act and fast-track designations have spurred drug development and availability. The advent of new drugs like Duvyzat and expedited clinical trials under the guidance of the FDA have made America a world leader in treating DMD.
The pricing analysis for Duchenne muscular dystrophy treatment involves a complex set of factors, including the cost of drugs, technology, regulatory expenses, insurance coverage, and regional differences in healthcare infrastructure. Below is a detailed breakdown of specific pricing factors that influence the Duchenne muscular dystrophy treatment market:
Drugs like eteplirsen (Exondys 51) and golodirsen (Vyondys 53) are among the leading exon-skipping therapies for DMD. The cost of these therapies can range from US$ 300,000 to US$ 400,000 per year per patient depending on the country and insurance coverage.
These treatments are costly due to the complex manufacturing processes involved, regulatory hurdles, and the limited patient population.
Certain treatments, like exon-skipping drugs, often require infusion, add significant costs due to healthcare professional involvement, hospital facilities, and equipment. The cost of an infusion can range from US$ 1,000 to US$ 10,000 per session, depending on the type of treatment.
Gene therapies typically require a one-time infusion, but the cost of the procedure and post-treatment care adds to the overall cost.
Patients receiving advanced treatments, particularly gene therapy and exon-skipping drugs, often require ongoing monitoring. Regular tests, including muscle biopsies, MRI scans, and genetic testing, add an additional US$ 5,000 to US$ 15,000 annually in clinical costs.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 8,988.7 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 47.85% | 2032 Value Projection: | USD 138,818.1 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer Inc., Fibrogen Inc., BioMarin, Santhera Pharmaceuticals, PTC Therapeutics, NS Pharma Inc., Nobelpharma Co. Ltd., Bristol-Myers Squibb, Sarepta Therapeutics, and Eli Lilly and Company |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Definition: Duchenne muscular dystrophy is a form of muscular dystrophy. It is caused by a defective gene for dystrophin (a protein in the muscles). It is a genetic disorder characterized by progressive muscle degeneration and weakness due to the alterations of dystrophin (a protein) that helps keep muscle cells intact.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients