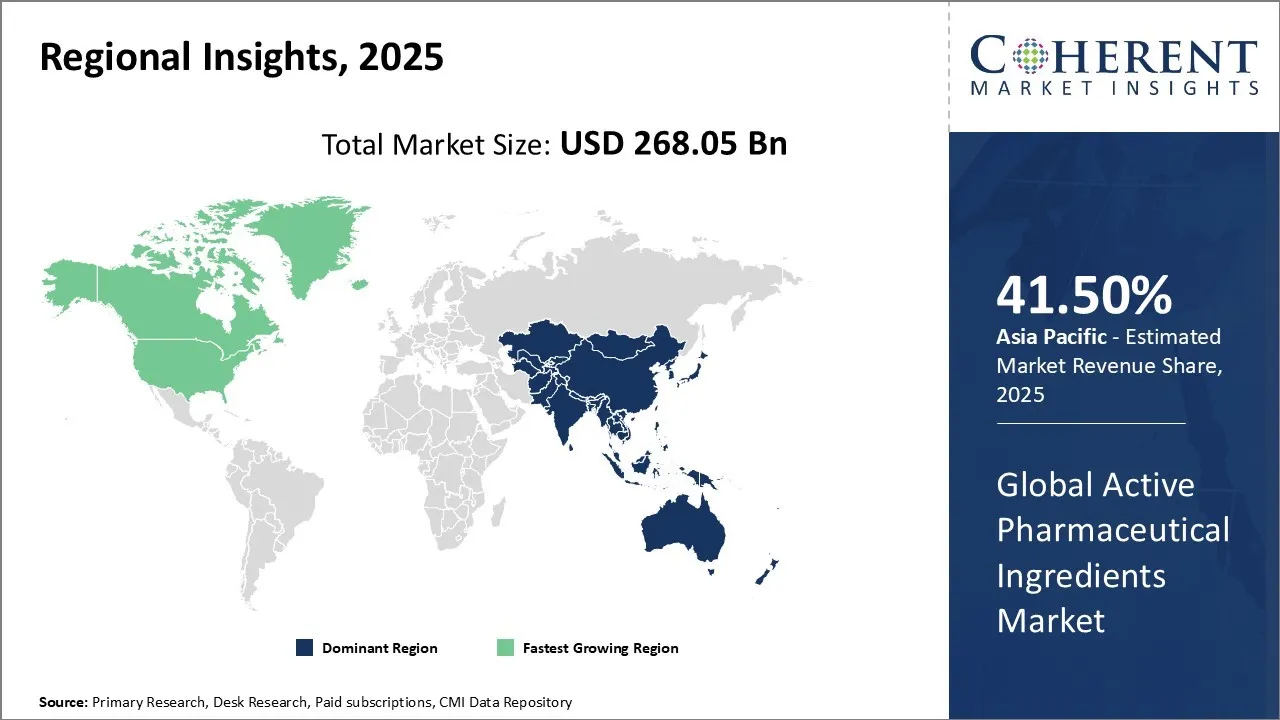
Active Pharmaceutical Ingredients Market is estimated to be valued at USD 268.05 Bn in 2025 and is expected to reach USD 424.83 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of6.8% from 2025 to 2032.
The active pharmaceutical ingredients market demand is experiencing robust growth, fueled by rising chronic diseases, biologics expansion, and outsourcing trends. Strong regulatory frameworks, technological advancements in synthesis, and cost-effective production in emerging economies are accelerating opportunities. Increasing global healthcare spending continues to drive active pharmaceutical ingredients market demand across therapeutic segments.
|
Current Event |
Description and its Impact |
|
EU Carbon Border Adjustment Mechanism (CBAM) and Environmental Regulations |
|
|
Biosimilar Market Expansion and Patent Cliff Impact |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Regulatory Compliance for APIs is a critical factor shaping the Active Pharmaceutical Ingredients (API) market. APIs must adhere to strict quality, safety, and efficacy standards set by regulatory authorities such as the FDA (U.S.), EMA (Europe), and PMDA (Japan). Compliance covers Good Manufacturing Practices (GMP), documentation, impurity profiling, stability testing, and batch consistency. Adherence ensures that APIs used in both generic and innovative drugs meet global market requirements, facilitating international trade and approval. Increasing regulatory scrutiny drives manufacturers to invest in advanced quality control systems and certification processes, thereby impacting production costs but ensuring Active Pharmaceutical Ingredients Market growth through trusted, high-quality products.
In terms of product type, the high potent APIs segment is projected to lead, accounting for 86.5% share in 2025 due to their critical role in advanced therapies. The surge is primarily driven by the rising prevalence of cancer, autoimmune disorders, and hormonal imbalances, where HPAPIs are integral to targeted and highly effective treatments. Their ability to deliver therapeutic effects at very low doses reduces side effects and enhances patient safety, making them essential for oncology drugs and biologics.
Additionally, the global shift toward precision medicine and biologically active compounds has accelerated HPAPI adoption. Growing R&D investments, patent expiries of blockbuster drugs, and the expansion of contract manufacturing organizations (CMOs) with HPAPI handling capabilities are further fueling this demand. As a result, HPAPIs dominate growth trends, positioning themselves as a key driver of innovation and competitiveness in the active pharmaceutical ingredients market.
For instance, in February 2025, SK pharmteco, a global CDMO, inaugurated a new CGMP-certified analytical testing laboratory dedicated to High-Potency Active Pharmaceutical Ingredients (HPAPIs).
In terms of molecular type, the small molecules segment is expected to contribute the largest share of the market in 2025, due to their cost-effectiveness, scalability, and established synthesis processes. They offer oral bioavailability, easier storage, and faster regulatory approvals compared to biologics. Rising chronic disease prevalence, patent expirations, and expanding generics further drive strong demand, reinforcing their critical role in global pharmaceutical development and accessibility.
For instance, in May 2025, Basel-based CDMO Lonza launched Design2Optimize™, a model-based platform that employs optimized design of experiments (DoE), predictive modeling, and digital twins developed with Fraunhofer ITWM to minimize physical testing, streamline reaction development, and speed small-molecule API timelines. The solution complements its AI-enabled route scouting and high-throughput experimentation tools.
In terms of formulation, the oral segment is projected to hold the highest share of the market in 2025, due to their ease of administration, affordability, and patient compliance. Widely used for chronic conditions, they are simpler to produce, store, and distribute than injectables. Advances in controlled-release and nano-formulations further strengthen demand, making oral APIs the most preferred formulation type globally.
For instance, in July 2025, Torrent Pharmaceuticals announced to launch both oral and injectable versions of semaglutide in India. The company is conducting Phase 3 clinical trials for the oral formulation and has partnered for injectable manufacturing. Torrent plans to produce the active pharmaceutical ingredient (API) in-house at its Gujarat facility. his strategic move aims to address the growing demand for GLP-1 therapies in diabetes and obesity treatment.
In terms of application, the cardiovascular segment is estimated to capture the greatest share of the market in 2025, due to the high prevalence of cardiovascular diseases (CVDs) worldwide, including hypertension, coronary artery disease, and heart failure. Aging populations, lifestyle changes, and rising risk factors such as obesity, diabetes, and sedentary behavior are driving long-term medication use, increasing demand for APIs in antihypertensives, anticoagulants, and lipid-lowering drugs. Additionally, continuous R&D and the introduction of combination therapies for improved efficacy are fueling growth. These factors collectively make the cardiovascular segment one of the largest contributors to API market demand, ensuring sustained consumption of high-volume, chronic-use active pharmaceutical ingredients.
For instance, in August 2025, Lupin Limited launched Bosentan Tablets for Oral Suspension (32 mg) in the U.S., following FDA approval of its partner NATCO Pharma’s Abbreviated New Drug Application. NATCO holds exclusive first-to-file status, granting it 180-day generic drug exclusivity. The product is a generic equivalent of Actelion’s Tracleer® and is indicated for treating pediatric pulmonary arterial hypertension.
To learn more about this report, Download Free Sample
Asia Pacific region is projected to lead the market with a 41.5% in 2025. The region offers cost-effective manufacturing capabilities and a large skilled workforce, making it an attractive hub for both generic and specialty API production. Rapidly growing pharmaceutical and biotech industries, coupled with increasing healthcare expenditures and rising prevalence of chronic diseases, are driving higher consumption of APIs. Supportive government policies, tax incentives, and investment in infrastructure further boost manufacturing and export activities. Additionally, the outsourcing trend among global pharmaceutical companies to Asia-Pacific countries, such as India and China, enhances regional production and supply chain dominance, contributing to the rising Active Pharmaceutical Ingredients Market demand in the region.
For instance, in September 2025, Sudarshan Pharma Industries Ltd enhanced its manufacturing capabilities by acquiring an operational Active Pharmaceutical Ingredient (API) facility in Telangana for ₹25.5 crore. The acquisition includes land, buildings, and plant machinery, facilitating the production of high-demand APIs such as Ropivacaine, Bupivacaine, and Sitagliptin. This strategic move aims to bolster the company's global market presence and regulatory compliance.
North America region is expected to exhibit the fastest growth in the active pharmaceutical ingredients (API) market with a 34.8% share in 2025. The region has a well-established pharmaceutical and biotechnology industry, with a high concentration of drug manufacturers, research institutes, and contract manufacturing organizations (CMOs). Rising prevalence of chronic and lifestyle-related diseases, including cancer, diabetes, and cardiovascular disorders, drives continuous demand for both generic and high-potency APIs. Strong regulatory frameworks enforced by the FDA ensure high-quality standards, encouraging domestic production and import of advanced APIs.
For instance, in September 2025, Lifecore Biomedical, a fully integrated contract development and manufacturing organization (CDMO), has announced its participation as a Gold Sponsor at the 24th Annual Contract Pharma Contracting and Outsourcing Conference. Lifecore will showcase its expertise in sterile injectable pharmaceutical products at Table 35 and engage with current and prospective clients to advance its growth strategy.
The United States is the largest market for APIs, driven by a strong pharmaceutical industry, high healthcare spending, robust R&D in biologics, generics, and specialty drugs, and strict FDA regulations ensuring high-quality standards.
For instance, in August 2025, AbbVie announced a $195 million investment to expand its active pharmaceutical ingredient (API) manufacturing at its North Chicago facility. This move is part of a broader $10 billion commitment to enhance U.S. innovation and manufacturing capabilities over the next decade. The new facility, set to begin construction in fall 2025 and become operational by 2027, will bolster production for neuroscience, immunology, and oncology medicines, supporting over 6,000 American jobs across 11 manufacturing sites.
India is a leading global exporter of generic APIs, supported by cost-effective manufacturing, skilled labor, and strong infrastructure, with rising domestic demand driven by chronic disease prevalence.
For instance, in September 2025, Aparna Pharmaceuticals achieved European Union Good Manufacturing Practice (EUGMP) certification for its Pydibhimavaram facility in Andhra Pradesh. This milestone, alongside the inauguration of a 7,300 sq. ft. R&D centre in Genome Valley, Hyderabad, underscores the company's commitment to global quality standards and innovation. The Pydibhimavaram site, already USFDA-approved, produces approximately 250 MT of APIs and intermediates monthly.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 268.05 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.8% | 2032 Value Projection: | USD 424.83 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Teva Pharmaceutical Industries Ltd., Pfizer, Inc., Dr. Reddy’s Laboratories Ltd., Novartis AG, Mylan N.V., Amneal Pharmaceuticals LLC, Lonza Group, Lupin Limited, Fresenius Kabi, Hikma Pharmaceuticals, Cipla Limited, Glenmark Pharmaceuticals Limited, Sun Pharmaceutical Industries Ltd., Endo International plc, Aurobindo Pharma Limited, Apotex Inc, Taro Pharmaceutical Industries Ltd, Stada Arzneimittel AG, Krka Pharmaceuticals, CordenPharma International, Evonik Industries AG, and Biological E. Limited. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The increasing prevalence of infectious diseases and chronic disorders is a major driver for the Active Pharmaceutical Ingredients (API) market size. As the incidence of conditions such as diabetes, cardiovascular diseases, cancer, and respiratory infections rises globally, the demand for both generic and specialty APIs escalates. Pharmaceutical companies are required to produce a wide range of APIs to develop effective therapeutic solutions, including high-potency and biologic APIs, to address these growing healthcare needs. This surge in disease burden not only expands the volume of API production but also stimulates innovation in drug delivery systems, directly contributing to the growth of the active pharmaceutical ingredients market size worldwide.
Increasing Demand for Generic Drugs is a major driver of Active Pharmaceutical Ingredients Market growth. As healthcare costs rise globally, both developed and emerging markets are turning to generic drugs as affordable alternatives to branded medications. This trend fuels consistent demand for high-quality APIs required for large-scale production of generics. Additionally, patent expirations of several blockbuster drugs are opening opportunities for generic drug manufacturers, further boosting API requirements. The shift toward cost-effective treatments in chronic and lifestyle-related diseases, coupled with government policies promoting generic drug use, strengthens production volumes and investment in API manufacturing. Overall, the rising preference for generics is directly propelling Active Pharmaceutical Ingredients Market growth worldwide.
The Active Pharmaceutical Ingredients (API) market is undergoing a significant transformation, driven by evolving geopolitical dynamics, technological advancements, and shifting industry priorities. These factors are reshaping the landscape of API production, distribution, and pricing, with profound implications for stakeholders across the pharmaceutical value chain.
A notable trend is the strategic reshoring of API manufacturing, particularly by U.S.-based pharmaceutical companies. Eli Lilly's commitment to investing $50 billion in building four new production facilities, including three dedicated to API production, underscores a broader industry movement towards domestic manufacturing. This initiative aims to mitigate supply chain vulnerabilities and reduce dependence on foreign sources, aligning with policy shifts favoring domestic production.
Similarly, Cambrex, a contract drug manufacturer, is seeking a $4 billion valuation for its sale, reflecting increased investor interest in U.S.-based pharmaceutical production. This interest is fueled by policy pressures, including potential tariffs on imported medicines and executive orders encouraging drugmakers to reshore manufacturing.
*Definition: Active pharmaceutical ingredients (APIs), also known as bulk pharmaceuticals, are the biologically active components in pharmaceutical drugs that produce the intended therapeutic effect. These ingredients are responsible for the pharmacological activity of a medication. APIs can be synthesized chemically or derived from natural sources such as plants, animals, or microorganisms, depending on the specific drug and its intended use.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients