Global Aldosterone Synthase Inhibitors Market Size and Forecast – 2025 to 2032
The Global Aldosterone Synthase Inhibitors Market is estimated to be valued at USD 213.2 Mn in 2025 and is expected to reach USD 611.7 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 16.3% from 2025 to 2032.
Key Takeaways of the Global Aldosterone Synthase Inhibitors Market
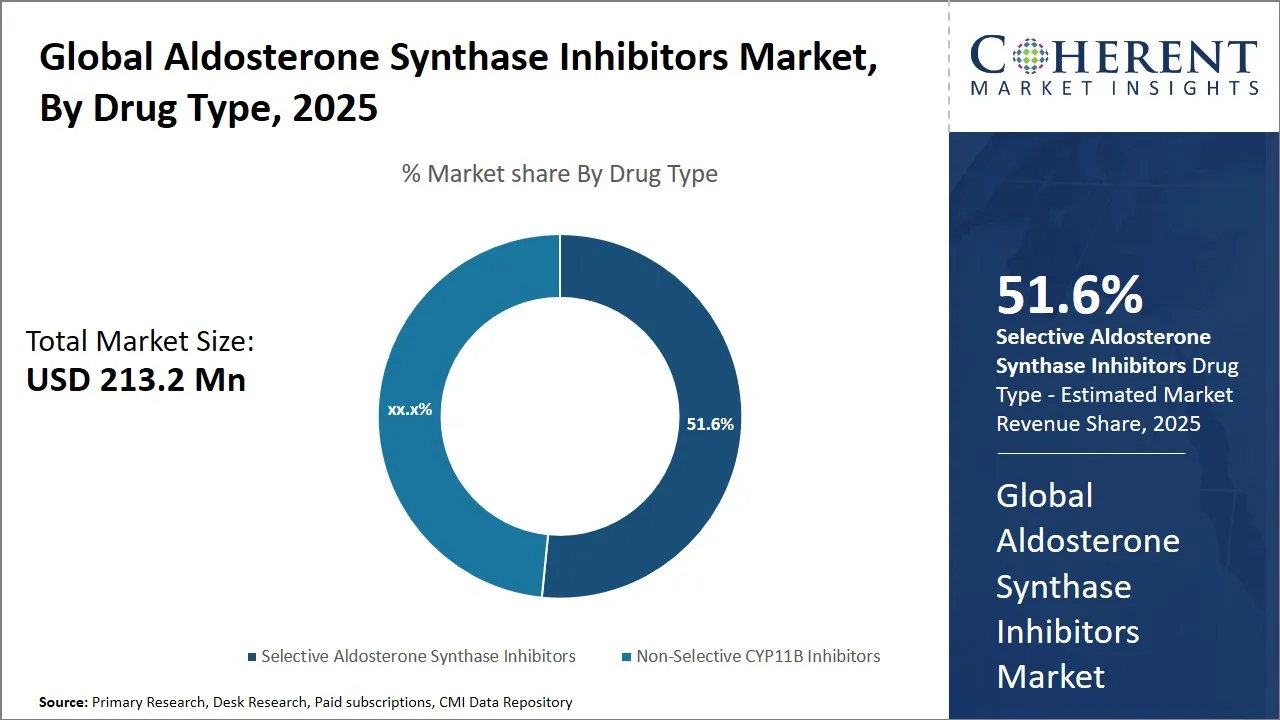
- Selective Aldosterone Synthase Inhibitors holds the largest market share by drug type, accounting for an expected 51.6% in 2025, due to the its superior efficacy in resistant hypertension.
- As for drug, the Osilodrostat segment is expected to dominate, contributing 63.5% to the global Aldosterone Synthase Inhibitors market in 2025, as it is the only approved drug (for Cushing’s syndrome), while competitors like Lorundrostat, Baxdrostat (Phase 3), Vicadrostat, and Dexfadrostat remain in clinical trials.
- In terms of indication, the Cushing’s Syndrome segment is expected to lead the market with a 21.3% share in 2025, as it is the only approved indication for Osilodrostat (LCI699), while other conditions like hypertension, primary aldosteronism, heart failure (HFrEF/HFpEF), and mHSPC remain investigational for aldosterone synthase inhibitors.
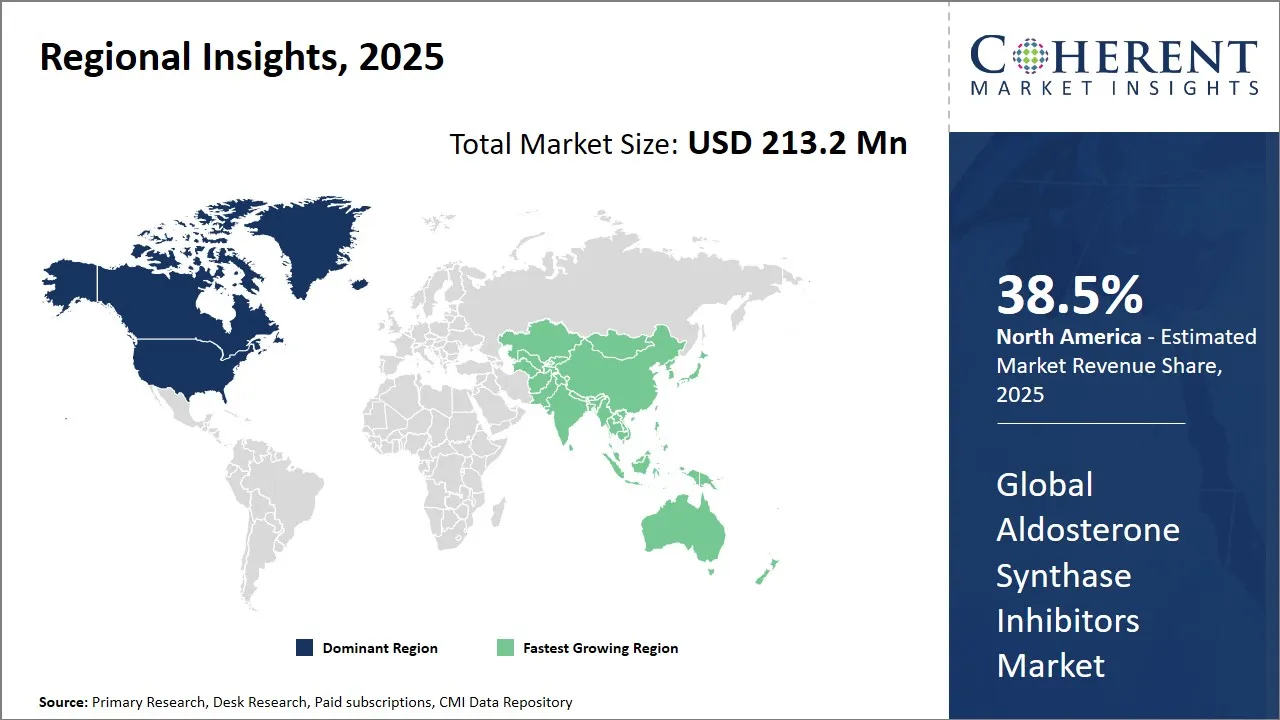
- North America is expected to lead the market, holding a share of 38.3% in 2025
- Asia Pacific is anticipated to be the fastest-growing region, with a market share of 25.7% in 2025.
Market Overview
The global aldosterone synthase inhibitors market is gaining traction as a promising therapeutic approach for conditions like hypertension, heart failure, and primary aldosteronism. Growth is driven by the need for targeted therapies beyond traditional mineralocorticoid receptor antagonists, with Osilodrostat being the only approved drug for Cushing's syndrome. Emerging selective inhibitors like Baxdrostat show potential for resistant hypertension, though safety concerns and high development costs remain challenges. The market's future hinges on clinical trial outcomes and expansion into new cardiovascular and endocrine indications.
Current Events and Its Impact
|
Current Events |
Description and its Impact |
|
Breakthrough in Hypertension Treatment: Mineralys Therapeutics Reports Positive Phase 2 Results for Lorundrostat in CKD Patients |
|
|
AstraZeneca Strengthens Cardiorenal Pipeline with Acquisition of CinCor Pharma and Baxdrostat |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Aldosterone Synthase Inhibitors Market Insights, By Drug Type – Selective Aldosterone Synthase Inhibitors Leads Due to the Its Superior Efficacy in Resistant Hypertension
The aldosterone synthase inhibitors market is currently dominated by selective aldosterone synthase inhibitors, which is expected to account for 51.6% of the total market share in 2025, significantly outpacing non-selective alternatives. This leadership position stems from their superior clinical efficacy, particularly in managing treatment-resistant hypertension, a condition where traditional therapies often fail. Unlike non-selective CYP11B inhibitors, which disrupt both aldosterone and cortisol pathways, selective inhibitors specifically target CYP11B2 (aldosterone synthase), minimizing off-target effects such as adrenal insufficiency.
Key drugs in this category, including baxdrostat (CinCor/AstraZeneca) and lorundrostat (Mineralys Therapeutics), have demonstrated strong blood pressure-lowering effects in clinical trials, reinforcing their therapeutic value. The precision of selective inhibitors also aligns with the growing demand for personalized medicine, as they offer a safer profile for long-term use in high-risk cardiovascular and renal patients. As a result, pharmaceutical development is increasingly focused on this subclass, with several candidates advancing through late-stage trials. The segment's dominance is expected to grow further as next-generation selective ASIs gain regulatory approvals and expand into additional indications like chronic kidney disease (CKD) and heart failure.
Aldosterone Synthase Inhibitors Market Insights, By Drug – Osilodrostat Leads as It is the Only Approved Drug (for Cushing’s syndrome) while Competitors like Lorundrostat, Baxdrostat (Phase 3), Vicadrostat, and Dexfadrostat Remain in Clinical Trials
The aldosterone synthase inhibitors market currently demonstrates a clear leader in Osilodrostat, which is expected to hold a commanding 63.5% market share in 2025 as the only U.S. FDA-approved drug in this class. Developed by Novartis, Osilodrostat has secured its first-mover advantage through its approval for Cushing's syndrome, establishing a strong foothold while competing candidates remain in various stages of clinical development.
This significant market dominance reflects both the high unmet need in Cushing's syndrome treatment and the current lack of approved alternatives. While promising competitors like Lorundrostat (Mineralys Therapeutics), Baxdrostat (AstraZeneca/CinCor), Vicadrostat, and Dexfadrostat are progressing through clinical trials for hypertension and other indications, none have yet achieved regulatory approval. Osilodrostat's position is further strengthened by its established safety profile and proven efficacy in managing cortisol excess, making it the go-to option for endocrinologists treating Cushing's patients.
Aldosterone Synthase Inhibitors Market Insights, By Indication – Cushing’s Syndrome is Solidifying its Position as It is the Only Approved Indication for Osilodrostat (LCI699)
The indication landscape for aldosterone synthase inhibitors reveals a clear market leader, with Cushing's syndrome solidifying its position as the dominant therapeutic application, capturing an estimated 21.3% of the total market share in 2025. This stronghold is primarily attributed to Osilodrostat (LCI699) being the only currently approved therapy in this drug class, specifically indicated for Cushing's syndrome. The drug's approval has created a well-defined treatment paradigm for this endocrine disorder, which is characterized by chronic cortisol excess.
While Cushing's syndrome maintains its established position, other potential indications remain in various stages of clinical investigation. Promising research is underway exploring applications in treatment-resistant hypertension, primary aldosteronism (Conn's syndrome), heart failure (both HFrEF and HFpEF variants), and metastatic hormone-sensitive prostate cancer (mHSPC). These emerging indications collectively represent significant future growth opportunities, though they currently lack approved therapies in the aldosterone synthase inhibitor category.
The market dynamics reflect the typical pharmaceutical development trajectory, where initial approvals in niche indications (like Cushing's syndrome) pave the way for potential expansion into broader cardiovascular and endocrine disorders. The 21.3% market share held by Cushing's syndrome treatments indicates both the current commercial success of Osilodrostat and the substantial untapped potential in other therapeutic areas. As clinical trials progress for other indications, this market segmentation is expected to evolve significantly, particularly if late-stage candidates like baxdrostat for hypertension receive regulatory approval.
Regional Insights
To learn more about this report, Download Free Sample
North America Aldosterone Synthase Inhibitors Market Analysis and Trends
The North America region is expected to hold the largest share which is expected to hold 38.3% in 2025 in the global Aldosterone Synthase Inhibitors market. The market is North America for aldosterone synthase inhibitors is positioned as the global leader, driven by several key factors including high prevalence of hypertension, strong healthcare infrastructure, and significant R&D investment in novel cardiovascular therapies. The region currently dominates market share, with the U.S. contributing the majority of revenue due to rising cases of resistant hypertension, primary aldosteronism, and Cushing's syndrome, conditions where aldosterone dysregulation plays a critical role.
The approval and commercialization of Osilodrostat (LCI699) for Cushing's syndrome has further solidified North America's position, while late-stage pipeline drugs like Baxdrostat (AstraZeneca) and Lorundrostat (Mineralys Therapeutics) for hypertension are expected to accelerate growth upon potential U.S. Food and Drug Administration approval. Challenges such as high treatment costs and stringent regulatory requirements persist, but the region’s robust clinical trial activity and favorable reimbursement policies support sustained market expansion. With a strong pipeline and growing unmet needs in cardiovascular and endocrine diseases, North America is expected to maintain its dominance in the aldosterone synthase inhibitors market through the forecast period.
Asia Pacific Aldosterone Synthase Inhibitors Market Analysis and Trends
The Asia Pacific region is expected to exhibit the fastest growth in the Aldosterone Synthase Inhibitors market with 25.7% of the market share in 2025, fueled by a rising epidemic of hypertension, cardiovascular diseases, and endocrine disorders. The disease burden in this region is particularly severe due to rapid urbanization, dietary shifts, with countries like China, India, and Japan reporting escalating cases of resistant hypertension, primary aldosteronism, and chronic kidney disease (CKD). Notably, China and India account for a significant portion of global hypertension cases, with studies indicating that nearly 30% of Asian hypertensive patients exhibit aldosterone excess, making them prime candidates for targeted aldosterone inhibition therapies.
The Asia Pacific region is witnessing significant disease trends that are driving growth in the aldosterone synthase inhibitors market. Hypertension and resistant hypertension represent critical health challenges, with the region having some of the highest prevalence rates globally. Poor disease control persists due to limited availability of advanced therapies, compounded by the fact that salt-sensitive hypertension, particularly prevalent in East Asian populations, is strongly influenced by aldosterone dysregulation.
Another key trend is the growing recognition of primary aldosteronism (Conn's Syndrome), which current estimates suggest may affect 5-10% of hypertensive patients across Asia. While historically underdiagnosed, increased clinical awareness is now spurring demand for specialized aldosterone testing and targeted treatments. The market is further propelled by the dual rise of chronic kidney disease and heart failure in the region. Diabetes-driven CKD has become particularly prevalent in South and Southeast Asia, where excessive aldosterone exacerbates renal damage.
Simultaneously, aging populations are experiencing higher rates of heart failure with preserved ejection fraction (HFpEF), creating new therapeutic needs for aldosterone-modulating drugs. These interconnected disease patterns underscore the expanding clinical and commercial potential for aldosterone synthase inhibitors across diverse APAC healthcare markets.
Global Aldosterone Synthase Inhibitors Market Outlook for Key Countries
U.S. Aldosterone Synthase Inhibitors Market Analysis and Trends
The U.S. remains at the forefront of this aldosterone synthase inhibitors market, supported by robust clinical research, a high prevalence of cardiorenal diseases, and early adoption of novel therapies like Osilodrostat, the only U.S. FDA-approved aldosterone synthase inhibitor currently indicated for Cushing's syndrome. For instance, on June 30, 2025, Mineralys Therapeutics, Inc., a clinical-stage biopharmaceutical company, focused on developing medicines to target hypertension, chronic kidney disease (CKD), obstructive sleep apnea (OSA) and other diseases driven by dysregulated aldosterone, announced the publication of the positive results from the pivotal Phase 3 Launch-HTN trial in the Journal of the American Medical Association (JAMA). Globally, the landscape is evolving with several late-stage candidates, such as AstraZeneca's baxdrostat and Mineralys Therapeutics' lorundrostat, targeting resistant hypertension and chronic kidney disease. The Launch-HTN trial evaluated the efficacy and safety of lorundrostat, a novel aldosterone synthase inhibitor (ASI), when added to existing background treatment in 1,083 participants with uncontrolled or treatment resistant hypertension. The trial demonstrated that lorundrostat significantly reduced systolic blood pressure (BP) with a favorable safety and tolerability profile.
These developments reflect a shift toward precision medicine, as selective aldosterone synthase inhibitors offer a more targeted alternative to traditional mineralocorticoid receptor antagonists (MRAs), minimizing side effects like hyperkalemia and gynecomastia. The market's growth is further fueled by increasing recognition of aldosterone's role in cardiovascular and metabolic diseases, alongside strategic industry investments in next-generation therapies.
Japan Aldosterone Synthase Inhibitors Market Analysis and Trends
Japan is emerging as a critical market for aldosterone synthase inhibitors, driven by its rapidly aging population and high burden of aldosterone-related disorders, including resistant hypertension, primary aldosteronism, and heart failure. The country's advanced healthcare system and emphasis on precision medicine create a favorable environment for the development and adoption of targeted therapies like selective aldosterone synthase inhibitors.
Key pharmaceutical players are actively pursuing strategies such as strategic funding, licensing agreements, and partnerships for clinical trial products to accelerate drug development and secure market positioning. Japan's well-established clinical trial infrastructure and efficient regulatory review processes further enhance its appeal as a testing ground for novel therapies. For instance, in April 2021, Mineralys Therapeutics, Inc., a private, clinical-stage biopharmaceutical company founded by Catalys Pacific announced today that the company completed a USD 40 million Series A funding round. The financing will enable Mineralys to complete its Phase 2 proof-of-concept study for MLS-101, as well as preparatory work for its pivotal trial development program. MLS-101 is a highly selective and potent aldosterone synthase inhibitor, licensed from Mitsubishi Tanabe Corporation, that is being investigated for the treatment of hypertension.
China Aldosterone Synthase Inhibitors Market Analysis and Trends
China is rapidly emerging as a pivotal market for aldosterone synthase inhibitors, fueled by its massive population with high rates of hypertension, cardiovascular diseases, and chronic kidney conditions linked to aldosterone dysregulation. The country's growing focus on innovative cardiovascular therapies has spurred both domestic and international pharmaceutical companies to accelerate clinical development in this space. Key players are actively advancing their pipelines through strategic milestones, including the successful dosing of the first subject in Phase I clinical trials for novel cardiovascular investigational products, signaling progress toward localized treatment options. For instance, in February 2024, Ji Xing Pharmaceuticals Limited, a clinical-stage biopharmaceutical company based in Shanghai, China, announced the successful dosing of the first subject in the Phase I clinical trial of the cardiovascular investigational product JX09 (formerly PB6440). JX09 is a next-generation, highly selective aldosterone synthase inhibitor.
India Aldosterone Synthase Inhibitors Market Analysis
India is emerging as a high-potential market for aldosterone synthase inhibitors, driven by the country's alarming rise in hypertension cases, particularly treatment-resistant forms linked to aldosterone imbalance. With one of the highest hypertension burdens globally, affecting nearly 30% of urban adults, India presents a critical need for advanced therapies that address underlying hormonal mechanisms.
The market is still in its early stages, with no approved aldosterone synthase inhibitors yet available, but growing awareness of primary aldosteronism (estimated in 5-10% of hypertensive Indians) is spurring clinical interest. According to the Global Burden of Disease 2019 (GBD 2019), prevalence of hypertension in India to be 22.6%, with men (24.1%) having a higher prevalence than women (21.2%). Domestic and multinational pharma companies are beginning to explore this space, encouraged by India's cost-effective clinical trial capabilities and a large, diverse patient pool ideal for research on resistant hypertension and cardiorenal diseases.
While infrastructure and diagnostic challenges persist, the government's push for non-communicable disease management and partnerships with global innovators could accelerate access to targeted therapies. As India's healthcare system increasingly recognizes aldosterone's role in cardiovascular risk, the foundation is being laid for future adoption of these precision medicines.
Aldosterone Synthase Inhibitors Target Epidemiology Database
- Cushing’s Syndrome (21.3% of ASI Use Cases)
- Global Prevalence: 40-50 cases/million, with approximately 13,000 diagnosed patients in the US (Endocrine Society 2024)
- Diagnosis Gap: 60% of cases remain undiagnosed for >3 years (EU Registry Data)
- Treatment Demand: 32% of patients fail first-line therapy, driving osilodrostat adoption
- Hypertension (Primary ASI Target)
- Resistant HTN: Affects 12-15% of 1.3B hypertensive patients globally (WHO 2024)
- Asia Dominance: China/India account for 48% of uncontrolled hypertension cases
- Aldosterone Link: 28% of resistant HTN cases show elevated aldosterone (PATHWAY-2 Trial Data)
- Primary Aldosteronism (Conn's Syndrome)
- Underdiagnosis: 5-10% of hypertensives affected, but <1% screened (JACC 2024)
- Japan Focus: Highest screening rates (12% of HTN workups) driving early ASI trials
|
Subtype |
Global Cases |
Aldosterone Role |
|
HFrEF |
26 Mn |
89% show aldosterone breakthrough |
|
HFpEF |
32 Mn |
76% with elevated RAAS activity |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
- Metastatic HSPC (Emerging Indication)
- Prevalence: 12% of 1.4 Mn annual prostate cancer cases
- Mechanism: Aldosterone shown to accelerate CRPC progression (NEJM June 2024)
- Trials: Vicadrostat preclinical data shows 41% tumor growth inhibition
Market Players, Key Developments, and Competitive Intelligence

To learn more about this report, Download Free Sample
Key Developments
- In February 2023, Ji Xing Pharmaceuticals Limited, a biopharmaceutical company announces the acquisition of global rights to an innovative asset in the cardiovascular field. Through the asset purchase, JIXING will obtain global rights to PB6440 from PhaseBio Pharmaceuticals, Inc. (PhaseBio). PB6440 is a novel drug candidate under preclinical development for patients with significant unmet medical needs including hypertension.
- In January 2020, PhaseBio Pharmaceuticals has signed an agreement with Viamet Pharmaceuticals and its wholly-owned subsidiary, Selenity Pharmaceuticals, to acquire all of the assets and intellectual property rights related to certain novel aldosterone synthase inhibitors, including the company’s lead development compound formerly known as SE-6440 or VT-6440. PhaseBio will designate the lead development compound as PB6440, which PhaseBio plans to develop for treatment-resistant hypertension.
Market Report Scope
Aldosterone Synthase Inhibitors Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 213.2 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 16.3% | 2032 Value Projection: | USD 611.7 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Mineralys Therapeutics, Inc., AstraZeneca, Boehringer Ingelheim International GmbH, DAMIAN PHARMA AG, CORXEL, JX09, Recordati Rare Diseases Inc., and Other Prominent Players |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Aldosterone Synthase Inhibitors Market Dynamics
To learn more about this report, Download Free Sample
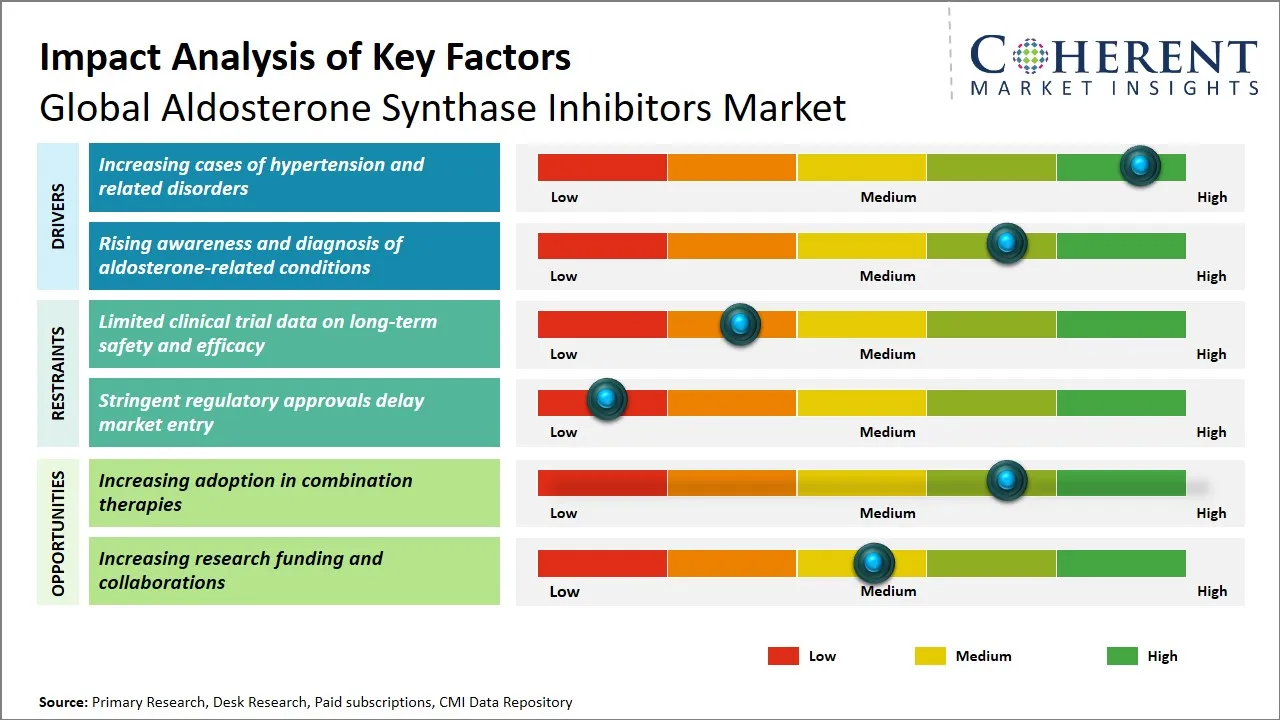
Aldosterone Synthase Inhibitors Market Driver - Increasing Cases of Hypertension and Related Disorders
The global prevalence of hypertension and its associated complications, such as heart failure, chronic kidney disease, and stroke, has been on a steady rise, significantly impacting the demand for effective therapeutic options like aldosterone synthase inhibitors. Aldosterone, a key mineralocorticoid hormone, is known to contribute to hypertension and promote adverse cardiovascular remodeling. As the burden of hypertension-related disorders grows there is an escalating need for targeted treatments that can effectively regulate aldosterone production.
According to the 1st WHO Global report 2023 on hypertension, hypertension remains a significant public health concern, contributing to the onset of cardiovascular diseases, stroke, and premature death. Only 54% of adults with hypertension are diagnosed, 42% receive treatment, and a mere 21% have their hypertension controlled. There are approximately 1.28 billion hypertension patients in the world. In US, nearly half of adults have hypertension (119.9 million) and only about 1 in 4 adults with hypertension have their hypertension under control (27.0 million). Many adults who are already treated with antihypertensive medication(s) may need to have their current medication dosage increased or to be prescribed additional medications to achieve blood pressure control (33.2 million). More than half of this group have blood pressure ≥140/90 mm Hg (18.8 million). Furthermore, there are approximately 245 million hypertension patients in China. Epidemiological surveys show that the awareness rate, treatment rate and control rate of hypertension in China are 51.6%, 45.8% and 16.8%, respectively, which are all relatively low.
Aldosterone synthase inhibitors specifically address this pathological mechanism by reducing aldosterone synthesis, thereby helping to control blood pressure and minimize organ damage. The increasing number of diagnosed patients who either exhibit resistance or contraindications to conventional antihypertensive therapies further accelerates interest and investment in these inhibitors, reinforcing their prominence in clinical management strategies for cardiovascular diseases driven by elevated aldosterone levels.
Aldosterone Synthase Inhibitors Market Opportunity - Increasing Research Funding and Collaborations
The aldosterone synthase inhibitors (ASI) market is witnessing a surge in opportunities, driven by increasing research funding and high-value collaborations across the pharmaceutical industry. With growing recognition of aldosterone's role in resistant hypertension, heart failure, and endocrine disorders, both public and private sectors are ramping up investments in ASI development.
Government grants, such as NIH funding for cardiovascular research, and venture capital backing for biotech startups are expanding the pipeline. Strategic partnerships are further fueling progress, AstraZeneca's acquisition of CinCor (for baxdrostat) and Mineralys Therapeutics' collaboration with Cleveland Clinic highlight the race to dominate this niche. Academic-industry alliances are also on the rise, with institutions like Mayo Clinic and European hypertension societies contributing to biomarker research and trial design. These collaborations not only de-risk development but also accelerate regulatory pathways, particularly for precision medicine applications in cardiorenal and metabolic diseases.
As more players enter the space, licensing deals and co-development agreements are expected to intensify, positioning ASIs as a high-potential drug class in the global cardiovascular market. The convergence of scientific innovation and strategic financing is set to unlock novel therapies, particularly for patients unresponsive to existing treatments.
Analyst Opinion (Expert Opinion)
- The aldosterone synthase inhibitors market is poised for significant growth, driven by rising demand for targeted cardiovascular therapies and increasing prevalence of resistant hypertension, primary aldosteronism, and Cushing’s syndrome. Technological advancements in precision medicine and selective CYP11B2 inhibitors, such as AstraZeneca’s baxdrostat and Mineralys Therapeutics’ lorundrostat, are accelerating clinical adoption. Regulatory support, including FDA Fast Track designations and EMA priority reviews, further bolsters market potential. However, challenges like high development costs, adrenal insufficiency risks, and competition from MRAs persist. Emerging opportunities lie in combination therapies (e.g., ASIs + SGLT2 inhibitors) and AI-driven dosing optimization, particularly in heart failure and chronic kidney disease.
- Key industry events like the American Heart Association (AHA) Scientific Sessions (2023–24) and European Society of Endocrinology (ESE) Annual Meetings have spotlighted ASIs, fostering collaborations and policy discussions. Notable initiatives include AstraZeneca’s acquisition of CinCor (baxdrostat) and expanded osilodrostat access programs in emerging markets. Pilot projects, such as Japan’s Sakigake-designated ASI trials, highlight regional efforts to expedite innovation. These developments underscore a dynamic market trajectory, with 2025–26 expected to bring pivotal approvals and commercial breakthroughs.
Market Segmentation
- Drug Type Insights (Revenue, USD Mn, 2020 - 2032)
- Selective Aldosterone Synthase Inhibitors
- Non-Selective CYP11B Inhibitors
- Drug Insights (Revenue, USD Mn, 2020 - 2032)
- Osilodrostat
- Lorundrostat
- Baxdrostat
- Vicadrostat
- Dexfadrostat
- Indication Insights (Revenue, USD Mn, 2020 - 2032)
- Cushing’s Syndrome
- Hypertension
- Primary aldosteronism (Conn's syndrome)
- Heart Failure
- Heart Failure with Reduced Ejection Fraction (HFrEF)
- Heart Failure with Preserved Ejection Fraction (HFpEF)
- Metastatic Hormone-sensitive Prostate Cancer (mHSPC)
- Stage of Development Insights (Revenue, USD Mn, 2020 - 2032)
- Preclinical
- Phase I Clinical Trials
- Phase II Clinical Trials
- Phase III Clinical Trials
- Approved or Marketed
- Gender Insights (Revenue, USD Mn, 2020 - 2032)
- Male
- Female
- End User Insights (Revenue, USD Mn, 2020 - 2032)
- Hospitals
- Cardiology Clinics
- Nephrology Clinics
- Research and Academic Institutes
- Contract Research Organizations (CROs)
- Regional Insights (Revenue, USD Mn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Mineralys Therapeutics, Inc.
- AstraZeneca
- Boehringer Ingelheim International GmbH
- DAMIAN PHARMA AG
- CORXEL
- JX09
- Recordati Rare Diseases Inc.
- Other Prominent Players
Sources
Primary Research Interviews
- Interviews with healthcare providers
- Interviews with key opinion leaders (KOLs)
- Interviews with pharmacists and medical professionals
- Interviews with patients using Aldosterone Synthase Inhibitors and other biologic therapies
Databases
- National Health Service (NHS) U.K.
- U.S. National Institutes of Health (NIH)
- Centers for Disease Control and Prevention (CDC)
- World Health Organization (WHO)
- European Medicines Agency (EMA)
- National Institute for Health and Care Excellence (NICE)
Magazines
- Pharmaceutical Technology
- BioPharma Reporter
- The Pharmaceutical Journal
Journals
- American Heart Association
- Journal of the American Heart Association (JAHA)
- Journal of Clinical Medicine
- Journal of Clinical Lipidology
- The New England Journal of Medicine
- Diabetology & Metabolic Syndrome
Newspapers
- The Guardian (UK)
- The New York Times
- The Financial Times
- The Washington Post
- The Times (UK)
- The Wall Street Journal
Associations
- Journal of the American Heart Association (JAHA)
- European Heart Journal (European Society of Cardiology - ESC)
- The Obesity Society
Public Domain Sources
- U.S. National Library of Medicine
- UK National Health Service (NHS) Resources
- National Health Interview Survey (NHIS)
- Centers for Disease Control and Prevention (CDC) Data
- National Institute for Health and Care Excellence (NICE) Guidelines
Proprietary Elements
- CMI Data Analytics Tool: Proprietary analytics tool to analyze real-time market trends, consumer behavior, and technology adoption in market
- Proprietary CMI Existing Repository of Information for Last 8 Years.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients