The antisense & RNAi therapeutics market size is expected to reach US$ 20.82 Billion by 2032, from US$ 7.59 Billion in 2025, at a CAGR of 15.5% during the forecast period. Antisense and RNA interference (RNAi) therapeutics work by inhibiting the expression of disease-associated genes and have shown great potential for the treatment of various medical conditions. Antisense therapy utilizes short strands of synthetic nucleic acids called antisense oligonucleotides (ASOs) that are designed to bind to and modulate mRNA, inhibiting target gene expression. ASOs can block transcription, RNA processing or transport, or translation. The main advantage of antisense therapy is that it allows precise downregulation of disease-relevant genes. However, a major challenge is the difficulty in delivering ASOs to tissue of interest.
RNAi therapeutics utilize small interfering RNAs (siRNAs) that mimic endogenous microRNAs (miRNAs) involved in post-transcriptional gene silencing. When introduced into the cell, siRNAs are incorporated into a multiprotein RNA-induced silencing complex (RISC) that guides the degradation of target mRNAs. Similar to ASOs, siRNAs can selectively inhibit the expression of target genes. The advantage of RNAi is its potency at modulating gene expression. However, challenges include the short half-life of siRNAs in circulation as well as difficulties in intracellular delivery. Despite obstacles, both antisense and RNAi therapeutics have shown promise for treating cancer, neurological disorders, and infectious diseases by selectively blocking genes driving disease pathology.
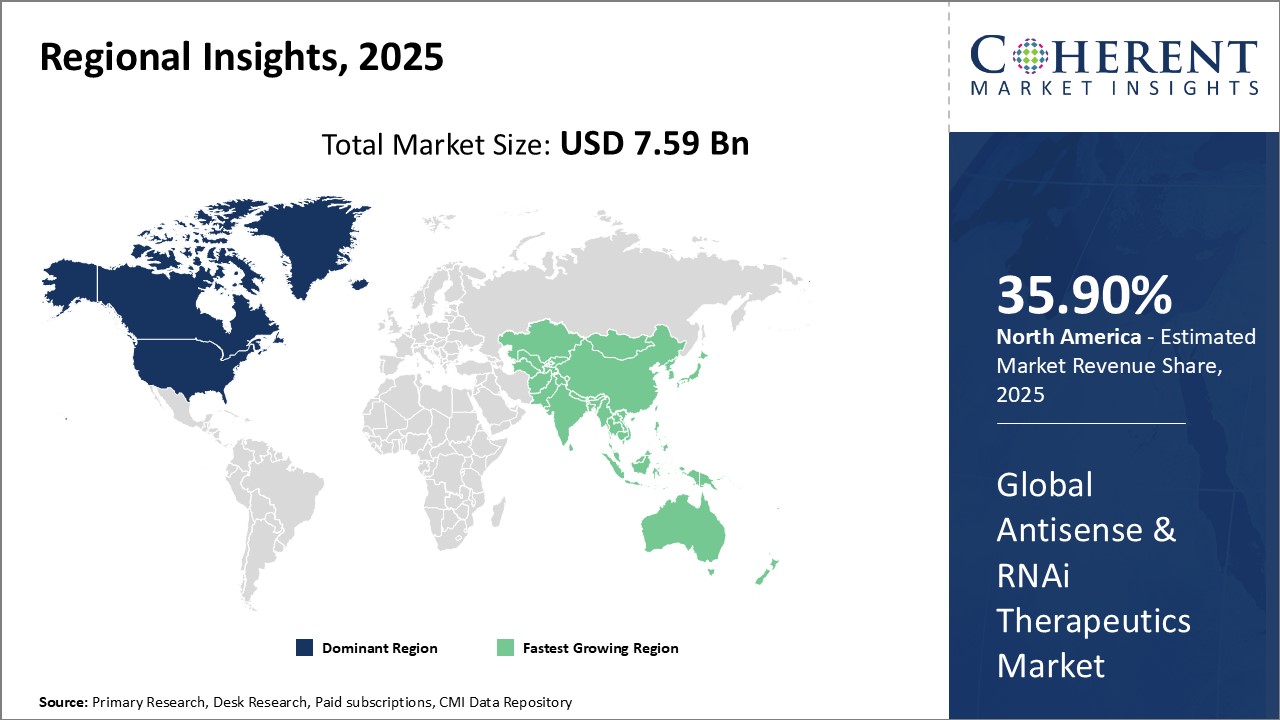
Antisense & RNAi Therapeutics Market Regional Insights
- North America is expected to be the largest market for antisense & RNAi therapeutics during the forecast period, accounting for over 35.9% of the market share in 2025. The growth of the market in North America is attributed to the strong presence of leading biopharma companies and world-class research institutions in countries like the United States. These companies have made massive investments in R&D efforts to develop innovative RNAi-based therapies. Additionally, the U.S. Food and Drug Administration has approved several antisense drugs in recent years, offering a boost.
- The Europe market is expected to be the second-largest market for antisense & RNAi therapeutics, accounting for over 28.5% of the market share in 2025. The growth of the market is attributed to the rising geriatric population and the increasing prevalence of diseases such as cancer and others.
- The Asia Pacific market is expected to be the fastest-growing market for antisense & RNAi therapeutics, with a CAGR of over 21.5% during the forecast period. The growth of the market in Asia Pacific is attributed due to Countries such as China, Japan, and India are rapidly enhancing their generic drug manufacturing capabilities as well as home-grown innovation. Several local biotech startups are exploring RNAi technologies for developing treatments. The lower manufacturing costs compared to Western markets are enabling these players to target therapies at affordable prices. This advantage is attracting sizable investments from global pharmaceutical leaders looking to expand their geographic footprints.
Figure 1. Global Antisense & RNAi Therapeutics Market Share (%), by Region, 2025
To learn more about this report, Download Free Sample
Analyst View
The global antisense & RNAi therapeutics market appears promising in the long run due to strong research and development investments from key players and increasing clinical trial activities. Major drivers will be the development of advanced nucleic acid-based drug molecules and the growing interest in precision medicines. Further clinical successes could accelerate revenue growth prospects over the next decade. Europe and North America currently lead global development and are likely to dominate the market in the near future owing to the concentration of leading pharmaceutical companies and the availability of late-stage pipelines. However, scientific and delivery challenges may restrain faster market uptake in the short-term. Efficient and safe delivery of nucleic acid therapeutics into target cells and tissues remains a key technical barrier. High R&D costs associated with new drug development also increase investment risks. Market players will need to focus on refining delivery technologies and biomarker strategies to better identify responsive patient populations. Regions like Asia are likely to see increased adoption driven by growing healthcare expenditures, expanding clinical trial activities, and strengthening intellectual property laws. Successful first-to-market products have potential to achieve blockbuster status given the current lack of effective therapies for many genetic diseases. Further consolidation in the space through partnerships, merger and acquisitions deals will help companies access new technologies and reduce risks. Overall, the market appears to be positioned for significant long-term
Antisense & RNAi Therapeutics Market Drivers:
- Growing Research and Development Investment: The increasing investment in R&D by pharmaceutical companies is a key factor responsible for driving the growth of the global antisense & RNAi therapeutics market. Over the past few years, companies have significantly ramped up their spending on research activities focused on developing innovative therapeutics based on RNA interference technologies. For instance, according to data from the Organization for Economic Co-operation and Development in April 2021, R&D expenditure in the pharmaceutical industry grew at an annual rate of 6.1% from 2015 to 2019, with total spending rising to over US$200 billion globally by 2020. This rise in R&D funds has enabled companies to explore RNAi and antisense drugs for treating various chronic and life-threatening diseases. Several biotech and pharma firms are conducting clinical trials to evaluate RNAi candidates against high-impact disease areas like cancer, cardiovascular disorders, metabolic diseases, neurological conditions, etc. For example, Alnylam Pharmaceuticals has multiple RNAi drugs in late-stage trials for treating primary hyperoxaluria and hereditary ATTR amyloidosis.
- Innovations in Drug Delivery Systems: The development of novel drug delivery systems to effectively deliver RNAi and antisense therapeutics has emerged as a major driver of market growth. Conventional delivery methods such as intravenous injection have posed significant bioavailability challenges due to rapid breakdown by nucleases in the bloodstream and difficulty in crossing cell membranes. However, advances in nanotechnology and material science have enabled the design of advanced carrier systems that can protect oligonucleotides from degradation and facilitate cellular internalization.
- Focus on Rare and Orphan Indications: While RNAi and antisense therapies hold promise for large markets like oncology, their initial clinical and commercial successes have predominantly been in rare and orphan disease indications. These include conditions like transthyretin amyloidosis (ATTR), spinal muscular atrophy (SMA), and familial chylomicronemia syndrome (FCS), which currently have very limited treatment options. For pharmaceutical companies, targeting such small patient populations with high unmet needs allows demonstrating early proof-of-concept and commercialization with relatively smaller trials. It also offers the potential for orphan drug designation and associated incentives, which make these rare disease programs strategically attractive. Several RNAi and antisense drugs have thus been approved or are in late-stage trials focused on rare genetic disorders. For instance, patisiran and inotersen gained FDA approval for treating hereditary ATTR. Additionally, companies continue to expand their pipelines with programs in other orphan realms like Duchenne muscular dystrophy, hemophilia, and Lysosomal storage diseases. As more of these niche pipeline assets receive approval, it will validate the utility of RNAi and antisense modalities. This success in rare diseases will, in turn, help incentivize their progress into larger mainstream indications. The growing industry efforts aligned towards orphan drug development thus represent a critical driver sustaining the growth of this therapeutics segment over the long term.
Antisense & RNAi Therapeutics Market Opportunities:
- Untapped emerging markets: Untapped emerging markets present a great opportunity for growth in the global antisense and RNAi therapeutics market. Many developing economies have large patient populations grappling with life-threatening diseases. However, current treatment options are limited or unavailable due to inadequate healthcare infrastructure and a lack of accessibility. Antisense and RNAi therapeutics have the potential to significantly improve patient outcomes in these countries by delivering cost-effective, targeted treatments. Having fewer side effects compared to conventional drugs, they can expand access to crucial medicines. Their application enables development of low-cost novel therapies for major diseases prevalent in emerging nations, like cancer, cardiovascular ailments, and hepatitis C, have high mortality. This ability to address the large unmet medical needs of impoverished populations can drive the market in untapped regions.
- New indications: The global antisense & RNAi therapeutics market has shown promising growth in recent years due to advancements in RNA-based drug development and greater understanding of molecular pathways involved in various diseases. This growing knowledge offers scope for these technologies to be applied to new disease indications beyond their current uses. Many pipeline drugs are being evaluated for conditions where treatment options are currently limited. If successful, these could open up entirely new areas for antisense and RNAi therapeutics to address. One such promising new avenue is in the field of neurodegenerative disorders. Diseases like Alzheimer's and Huntington's remain difficult to treat effectively. However, research increasingly points to RNA interference playing a role in the pathogenesis of certain neurodegenerative conditions.
Global Antisense & RNAi Therapeutics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 7.59 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 15.5% | 2032 Value Projection: | USD 20.82 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Benitec Biopharma Inc., Silence Therapeutics, Ionis Pharmaceuticals, Inc., Bio-Path Holdings Inc., Percheron Therapeutics Limited, GSK plc, Olix Pharmaceuticals, Inc., Sanofi, Alnylam Pharmaceuticals, Inc. and Arbutus Biopharma |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Antisense & RNAi Therapeutics Market Trends:
- Focus on personalized medicines: The global antisense and RNAi therapeutics market is experiencing significant influence from the growing trend of multidisciplinary development approaches. Conventional drug development typically involved separate silos for discovery, preclinical and clinical research, and regulatory functions. However, there is now a shift towards more integrated, multidisciplinary models that bring together diverse expertise throughout the entire drug development life cycle. This holistic, collaborative approach is proving beneficial for antisense and RNAi therapies, which present unique technical challenges. By their very mechanism of action, these nucleic acid-based drugs require input from a wide breadth of scientific knowledge, including molecular biology, biochemistry, pharmacology and clinical medicine. Multidisciplinary teams allow these specialized skillsets to collectively advance antisense and RNAi candidates in a more streamlined manner. For example, researchers from Ionis Pharmaceuticals published results in 2022 demonstrating their cross-functional development model helped accelerate delivery of a new antisense drug to treat hereditary transthyretin amyloidosis by 2 years compared to traditional approaches.
Antisense & RNAi Therapeutics Market Restraint:
- High Manufacturing Cost of RNA Therapeutics: One key challenge restraining rapid market expansion is the high costs involved in manufacturing RNA therapeutics such as antisense oligonucleotides and siRNA molecules. The production of these sequence-specific nucleic acid drugs using synthetic processes is quite complex and expensive. It requires specialized equipment and multi-step chemical synthesis which makes the overall manufacturing process capital intensive. Companies need to incur high capital expenditure to establish robust infrastructure for industrial-scale production of RNA drugs. This adds significantly to the drug development costs. Further, production inconsistency and impurities also impact the manufacturing economics. The high costs ultimately increase the drug prices, reducing affordability and market uptake to some extent. Overcoming the economic barriers of manufacturing remains a major restraint area for the antisense & RNAi therapeutics market.
- Delivery Challenges Associated with RNA Therapeutics: Another key challenge is the efficient delivery of RNA therapeutic compounds to target tissues and intracellular sites of action inside the body. Naked RNA molecules face rapid degradation and do not efficiently cross cell membranes to elicit pharmacological activity. This necessitates the use of delivery technologies like nanoparticles, liposomes, cell-penetrating peptides, etc. to protect RNA drugs and facilitate uptake. However, developing safe and effective delivery systems that can withstand biological environment remains difficult. Issues like improper tissue-tropism of carriers, immune reactions, reliability of encapsulation, & release mechanisms continue to hinder the delivery potential of RNA drugs.
Recent Developments
New product launches
- In April 2023, Alnylam Pharmaceuticals, Inc., a pharmaceutical company, and Regeneron Pharmaceuticals, Inc., a pharmaceutical company, announced positive interim results from the ongoing single-ascending portion of the Phase 1 trial of ALN-APP, an experimental RNAi therapeutic amyloid -Forerunne, targeting protein (APP) in development for the treatment of Alzheimer's disease and cerebral amyloid angiopathy (CAA).
- In March 2023, OliX Pharmaceuticals, Inc., a pharmaceutical company, the first patient dosage in a Phase 1 clinical study marked a significant milestone. This study focuses on a groundbreaking RNAi therapy that has the potential to prevent and treat age-related macular degeneration (AMD).
Acquisition and partnerships
- In December 2022, GSK plc, a pharmaceutical company, and Wave Life Sciences Ltd., a clinical-stage genetic medicine company committed to providing life-changing treatments for people with devastating diseases, announced a strategic collaboration to advance oligonucleotide therapeutics, including Wave's preclinical RNA editing program, Combating Alpha-1 Antitrypsin Deficiency (AATD), WVE-006. The research cooperation initially has a four-year research period. It combines GSK's unique human genetics insights, global development, and commercial capabilities with PRISMTM, Wave's proprietary discovery and drug development platform.
- In June 2020, Evox Therapeutics Ltd., a leading exosome therapeutics company, is pleased to announce a research collaboration and licensing agreement with Eli Lilly and Company to develop the proprietary DeliverEX RNA interference (RNAi) platform from Evox to develop and use antisense oligonucleotide (ASO) drug payloads for the potential treatment of neurological disorders.
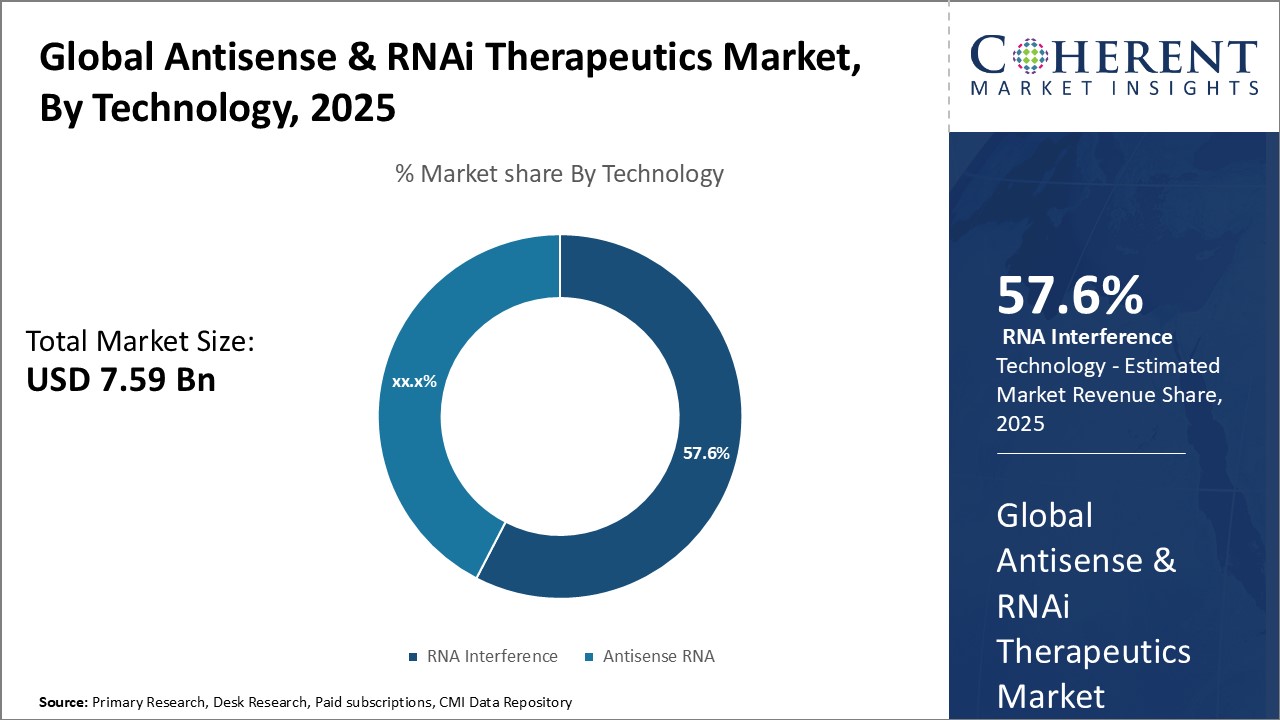
Figure 2. Global Antisense & RNAi Therapeutics Market Share (%), By Technology, 2025
To learn more about this report, Download Free Sample
Top companies in Antisense & RNAi Therapeutics Market
- Benitec Biopharma Inc.
- Silence Therapeutics
- Ionis Pharmaceuticals, Inc
- Bio-Path Holdings Inc.
- Percheron Therapeutics Limited
- GSK plc
- Olix Pharmaceuticals, Inc.
- Sanofi
- Alnylam Pharmaceuticals, Inc.
- Arbutus Biopharma
Definition: Antisense and RNAi therapeutics are types of gene therapies in which RNA molecules are used to silence or change the expression of specific genes. They have been used to treat a variety of illnesses, including cancer, cardiovascular disease, and uncommon genetic disorders.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




