Global Sacral Neuromodulation Market Size and Forecast – 2025 to 2032
The global sacral neuromodulation market is estimated to be valued at USD 1.91 Bn in 2025 and is expected to reach USD 4.07 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 11.4% from 2025 to 2032. This robust growth reflects increasing adoption of neuromodulation technologies for managing various pelvic and urinary disorders, driven by advancements in device innovation and expanding patient populations worldwide.
Key Takeaways of the Global Sacral Neuromodulation Market
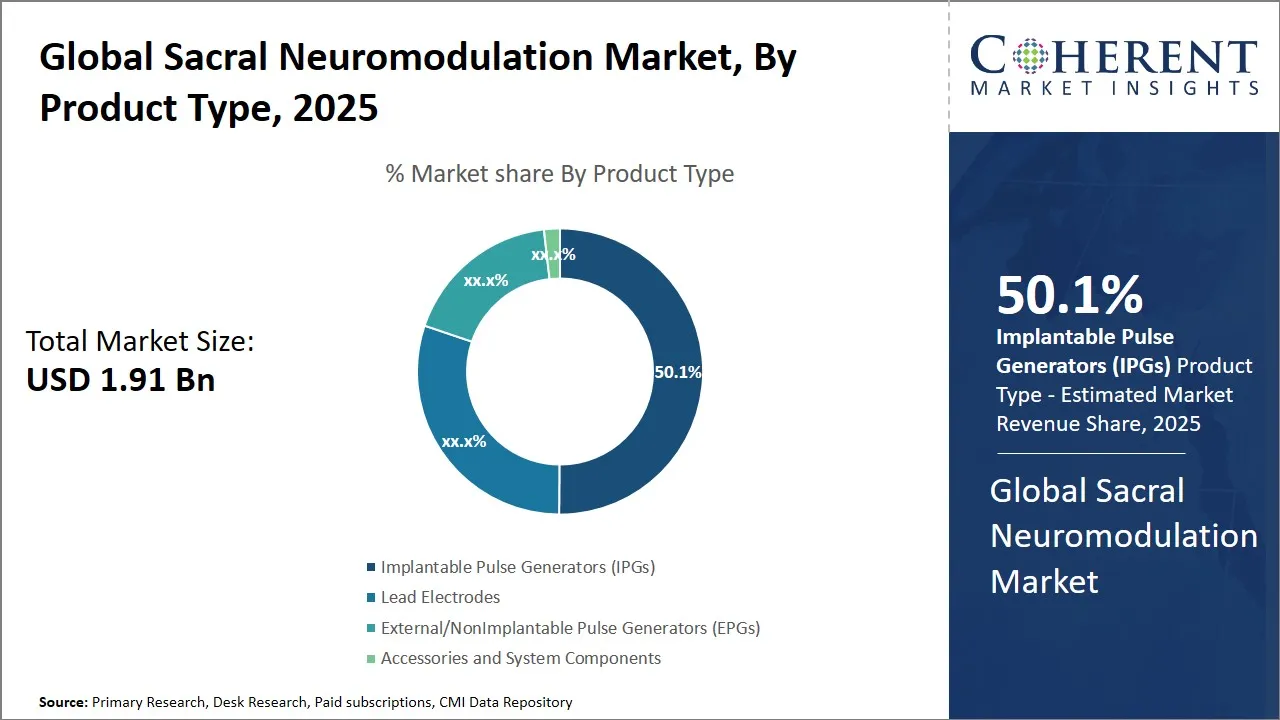
- In 2025, Implantable Pulse Generators (IPGs) segment is expected to dominate the global sacral neuromodulation market, holding the largest share at 50.1 %.
- Among application, the urinary incontinence (stress, urge, mixed) segment is projected to capture the highest market share at 24.5 %.
- In the end user, the hospitals and large medical centers segment is anticipated to lead, accounting for 34.2% of the market share in 2025.
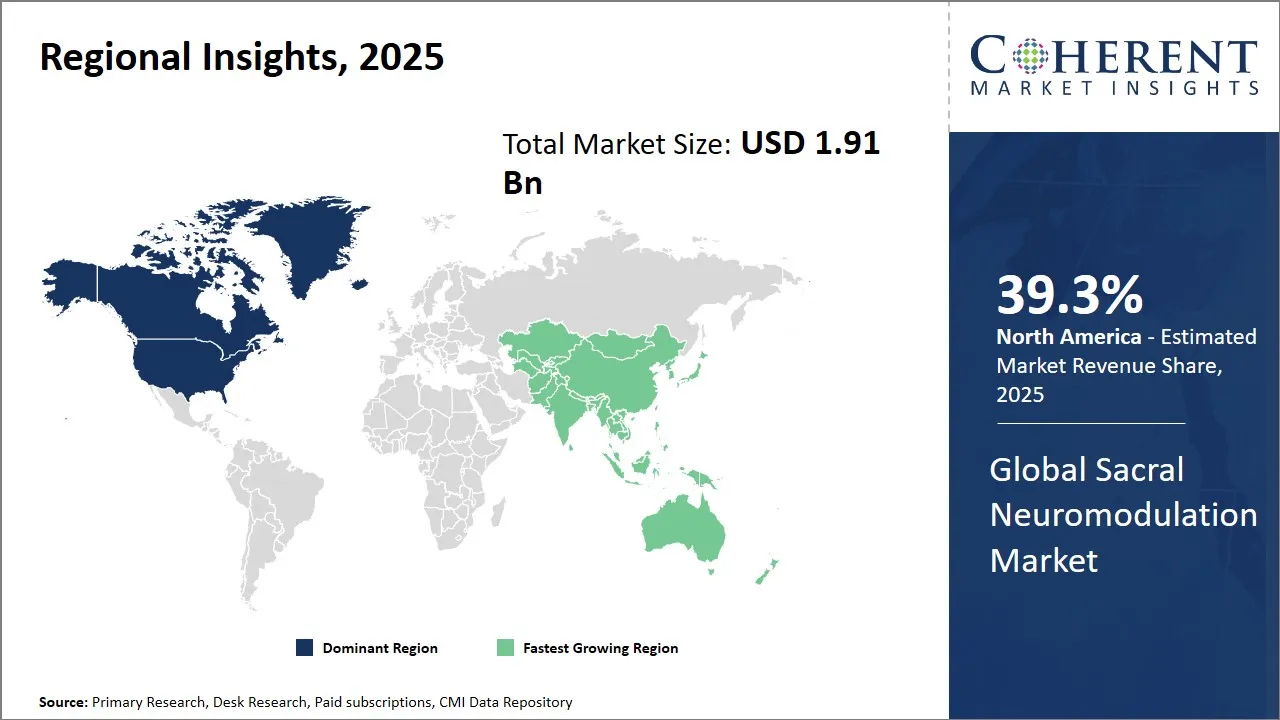
- North America is expected to lead the market, holding a share of 39.3% in 2025. Asia Pacific is anticipated to be the fastest-growing region, with an estimated market share of 24.6% in 2025.
Market Overview
Key trends shaping the sacral neuromodulation market include integration of smart and wireless technologies to enhance device functionality and patient comfort. Additionally, growing awareness among healthcare providers and patients about minimally invasive surgery options is driving the market expansion. Increasing investments in research and development are leading to improved efficacy and safety profiles, while rising prevalence of urinary and bowel dysfunctions globally further propels the demand for sacral neuromodulation therapies.
Current Events and Its Impact
|
Current Events |
Description and its Impact |
|
Accelerated Adoption of AI and Digital Healthcare Platforms |
|
|
Rapid technology advances: wireless, MRI ‑ compatible and rechargeable sacral neuromodulation systems |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Sacral Neuromodulation Market Insights, By Product Type - Implantable Pulse Generators (IPGs) Lead Product Innovation and Market Dominance
Implantable Pulse Generators (IPGs), as a product type, take the lead in the global sacral neuromodulation market with an estimated 50.1% share in 2025, due to their essentiality in the application of various pelvic floor disorders therapy over a long period. IPGs, the heart of the sacral neuromodulation systems, to control nerve activity, and treat such conditions as urinary incontinence and overactive bladder, are pushed out with the electric pulses.
In November 2022, CVRx, a company specializing in U.S. FDA-approved neuromodulation devices, launched the Barostim Neo2 implantable pulse generator (IPG) to treat heart failure symptoms. The new device is 10% smaller and offers 20% longer battery life, reducing the frequency of replacements for patients. It also features a streamlined design with a single lead port, simplifying the implant procedure.
Sacral Neuromodulation Market Insights, By Application - Urinary Incontinence (stress, urge, mixed) Leads Application Demand Due to Rising Prevalence and Awareness
The largest share of urinary incontinence (stress, urge, mixed) in sacral neuromodulation applications, is estimated at 24.5% share in 2025, mainly because of its high incidence rate and the treatment’s effectiveness in controlling the symptoms.
The global rise in urinary incontinence, especially among the elderly and women after childbirth, points to hidden demand of powerful therapeutic alternatives that are not just confined to the pharmaceutical or behavioral sector, which are often compromising with success or making the patient suffer with side-effects.
Sacral Neuromodulation Market Insights, By End User - Hospitals and Large Medical Centres Drive Market Through Advanced Treatment Delivery
The hospitals and large medical centers segment is projected to represent the largest end user share of the sacral neuromodulation market with 34.2% in 2025, mainly owing to their outstanding medical infrastructure, workforce and ability to provide sophisticated surgical operations for the insertions of devices and post-operative care.
Such places usually have the operating rooms especially designed for the surgeries, the imaging technology, and the trained urologists, neurologists, and pelvic floor specialists who are the ones to work together in managing the patient pathways through the complexities that come along with it thus, ensuring high success rates and patient safety.
Technological Advancements in Sacral Neuromodulation: (MRI Compatibility and Rechargeable Systems)
- Technological advancements in the sacral neuromodulation market have significantly enhanced treatment options for patients. Devices like the Axonics Sacral Neuromodulation System have introduced MRI compatibility, allowing patients who require regular MRIs to continue using sacral neuromodulation therapy without the need to remove the device. This breakthrough has opened up the market to a broader patient base, particularly those with conditions like cancer or musculoskeletal issues, which often necessitate MRI scans.
- Additionally, rechargeable systems, such as the Axonics device, offer a major improvement over traditional sacral neuromodulation devices, like Medtronic’s InterStim, which required battery replacements every 5 to 7 years. The Axonics system’s rechargeable battery can last up to 15 years, reducing the need for multiple surgeries and improving patient convenience. These technological innovations not only extend the lifespan of the device but also enhance the overall patient experience, making sacral neuromodulation a more accessible and sustainable option for treating conditions like overactive bladder and fecal incontinence.
Regional Insights
To learn more about this report, Download Free Sample
North America Sacral Neuromodulation Market Analysis and Trends
North America will still be the leading region in the global market for sacral neuromodulation devices, with a share of 39.3% in 2025. A well-established healthcare system, a considerable number of top-notch medical device firms, and lenient regulatory policies that ease product approvals are the main factors behind this leading position.
Investment in public and private sectors in the USA and Canada aimed at urology and neurology research is also huge and, in this way, it helps the innovation to promote the usage of advanced neuromodulation therapies. Furthermore, the key industry players like Medtronic, Axonics Modulation Technologies, and Boston Scientific are the reasons behind the product development and the widespread clinical application of the technology.
Asia Pacific Sacral Neuromodulation Market Analysis and Trends
The Asia Pacific sacral neuromodulation market is rapidly expanding, prompted by the increasing demand awareness with an estimated share of 24.6% in 2025, improved healthcare infrastructure, and rising healthcare expenditure in countries like China and India. The government regulations in different countries are also making things easier for realizing the full potential of chronic disease management with respect to overactive bladder and fecal incontinence.
The partnerships between the multinational medical device firms and the local distributors like Medtronic and LivaNova are speeding up the technology acceptance process. The facilitations in reimbursement policies and the openness in trade are also among the factors that are propelling the market growth.
Sacral Neuromodulation Market Outlook for Key Countries
U.S. Sacral Neuromodulation Market Analysis and Trends
The U.S. healthcare system remains the most important factor in the sacral neuromodulation market, thanks to the high healthcare expenditure, advanced clinical trials, and the speed with which medical technologies are adopted. The major companies Medtronic and Boston Scientific have a firm grip on the market by constantly upgrading their implantable devices to boost patient outcomes. The strong reimbursement system encourages doctors even more to recommend these kinds of therapies.
In March 2022, Valencia Technologies, a privately held company, announced the FDA approval of its eCoin leadless tibial neurostimulator for treating urinary urge incontinence (UUI), a common symptom of Overactive Bladder (OAB). The eCoin device, about the size of a nickel, is implanted subcutaneously in the lower leg through a minimally invasive procedure.
Germany Sacral Neuromodulation Market Analysis and Trends
Germany is known for its excellent healthcare system, strict regulations, and a strong medical device manufacturing sector, all of which contribute to the sacral neuromodulation market. The government has introduced policies that support the use of advanced technologies for the treatment of chronic urological diseases, which, along with increased healthcare funding, are factors that drive the market. In addition, Germany has a high level of physician expertise and patient awareness which are the main reasons for the constant demand for neuromodulation therapies.
Medtronic is a major player in the sacral neuromodulation market and is located in Germany. The company’s InterStim system, which is designed for chronic pelvic pain, overactive bladder, and fecal incontinence, has been widely accepted in the country.
China Sacral Neuromodulation Market Analysis and Trends
China is a rapidly growing sacral neuromodulation market characterized by increased patient awareness, a rise in related chronic conditions, and better accessibility to healthcare. The government's plans to modernize hospitals and promote innovative medical technologies have a positive impact on market growth. Additionally, reforms in regulations designed to speed up the approval process for medical devices have resulted in quicker market entry and have thus reinforced China's position as a leading growth driver in the Asia Pacific region.
Boston Scientific has been making great progress in China sacral neuromodulation market. Their groundbreaking Precision system, which is intended for patients who suffer from bladder and bowel dysfunction, is becoming more and more accepted because of the government's backing of medical technology and the lifting of healthcare infrastructure.
Japan Sacral Neuromodulation Market Analysis and Trends
Japan, on the other hand, continues to be the leading country in the Asia Pacific region with the advantage of a mature healthcare system, high per capita healthcare expenditure, and clinical acceptance of the neuromodulation devices. The country has a number of clinical centers that are exclusively engaged in sacral neuromodulation procedures and these are further supported by the government health insurance which covers such innovative treatments.
Uroplasty, a division of Endo International, is a company that has a presence in Japan, and that has its product Urgent PC Neuromodulation System gaining ground. This system is used for the treatment of urinary incontinence and pelvic floor disorders and enjoys a high level of acceptance in Japan due to the strength of the healthcare system and the availability of insurance coverage.
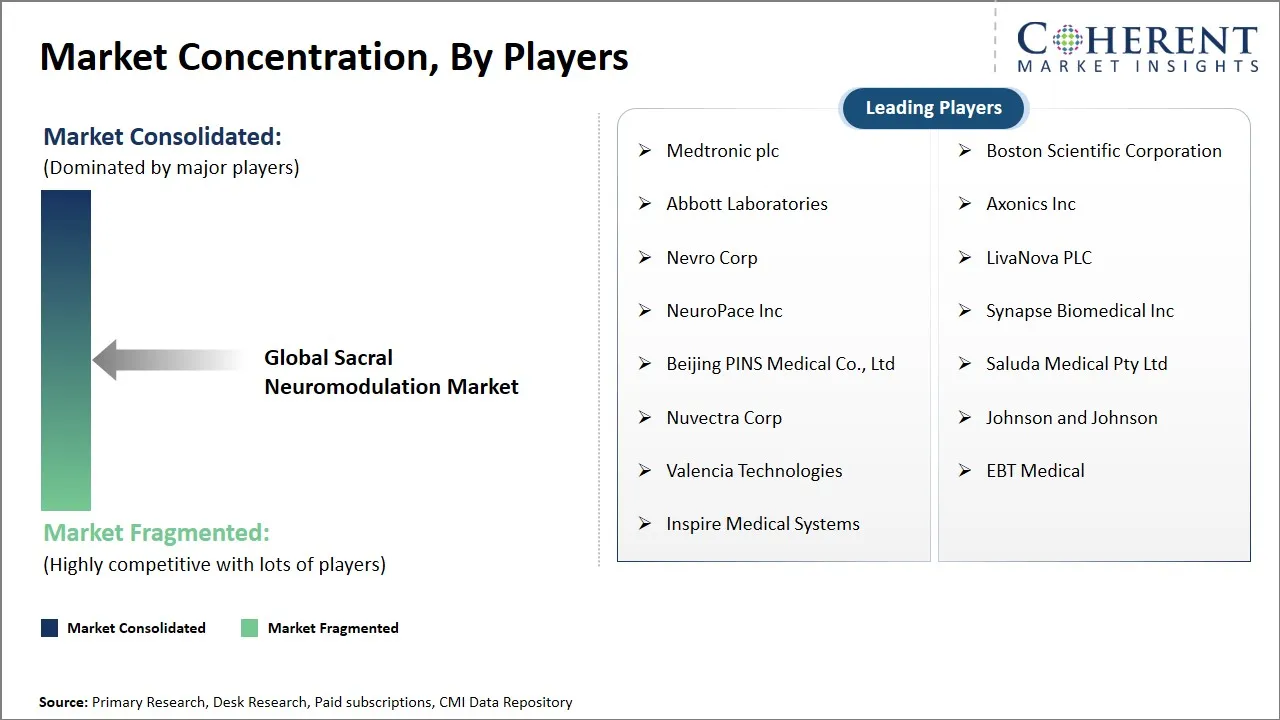
Market Players, Key Developments, and Competitive Intelligence
To learn more about this report, Download Free Sample
Key Developments
- In September 2025, Boomerang Medical raised USD 20 million in Series B funding to advance its pivotal trial, BOOM-IBD2, for sacral neuromodulation in ulcerative colitis. The trial will evaluate the potential of neuromodulation to reduce symptom burden in patients with this debilitating form of inflammatory bowel disease.
- In June 2025, Neuspera Medical, a leading developer of integrated neuromodulation technologies, announced FDA approval for its integrated sacral neuromodulation (iSNM) system, designed to treat urinary urge incontinence (UUI). The iSNM system offers patients a non-invasive alternative to traditional therapies, eliminating discomfort and risks associated with implanted batteries.
- In January 2023, Axonics received U.S. FDA approval for its fourth-generation rechargeable sacral neuromodulation system, the Axonics R20. The device offers a 20-year lifespan and extends recharge intervals to just once every 6 to 10 months, enhancing patient convenience. Axonics plans to launch the R20 in March 2023, marking a significant advancement in treating overactive bladder and fecal incontinence.
- In July 2022, a study published in New England Journal of Medicine Evidence (NEJM Evidence) showed that sacral neuromodulation (SNM) effectively treated neurogenic lower urinary tract dysfunction (NLUTD). Conducted in Switzerland, the trial demonstrated that SNM improved bladder function in patients with refractory NLUTD, with 76% of those receiving SNM showing positive results. This highlights the potential of SNM in treating urinary dysfunctions related to neurological conditions.
Top Strategies Followed by Global Sacral Neuromodulation Market Players
- The sacral neuromodulation market has a strategy of innovating and expanding their global outreach to conquer the competition. They lavishly spend on research and development (R&D) to build up-to-the-minute, super-performance devices that cater to the needs of various patients. Besides, these giant companies are proactively forming strategic partnerships with original equipment manufacturers (OEMs), healthcare institutions, and tech companies, thereby drawing their product range and reinforcing their position in the market.
- In January 2024, Boston Scientific announced its agreement to acquire Axonics, Inc., a medical technology company specializing in devices for urinary and bowel dysfunction. The acquisition, valued at approximately USD 3.7 billion, aims to expand Boston Scientific’s urology portfolio with Axonics' advanced sacral neuromodulation (SNM) systems, including the Axonics R20 and F15 systems.
- The mid-level competitors are implementing a different tactic by being the ones to provide cheaper solutions and focusing on such consumers who are sensitive to the price, especially in the underdeveloped areas. They are the ones who are striving for quality and affordability to not disturb performance, thereby wanting to take a major portion of the market that is budget-conscious.
- Stimwave is an example of a mid-level player offering affordable neurostimulation solutions, balancing quality and cost. The company has expanded its reach through strategic collaborations with distributors in emerging markets.
- The small-scale competitors are paying attention to the low-demand sectors by using cutting-edge technologies such as microelectronics and smart sensing systems to come up with their own specialized products. They are the ones who are pouring their creativity into meeting the clinical needs through their products. Also, they are the ones who are collaborating with local startups, and the like, for the partnership to enhance their market visibility and getting the regulatory approvals quicker.
- Neuspera Medical focuses on advanced, small-scale sacral neuromodulation devices. It partners with regional manufacturers and healthcare providers to gain regulatory approvals and increase market visibility in niche segments.
Market Report Scope
Sacral Neuromodulation Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.91 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.4% | 2032 Value Projection: | USD 4.07 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medtronic plc, Boston Scientific Corporation, Abbott Laboratories, Axonics Inc, Nevro Corp, LivaNova PLC, NeuroPace Inc, Synapse Biomedical Inc, Beijing PINS Medical Co., Ltd, Saluda Medical Pty Ltd, Nuvectra Corp, Johnson and Johnson, Valencia Technologies, EBT Medical, and Inspire Medical Systems |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Sacral Neuromodulation Market Dynamics
To learn more about this report, Download Free Sample
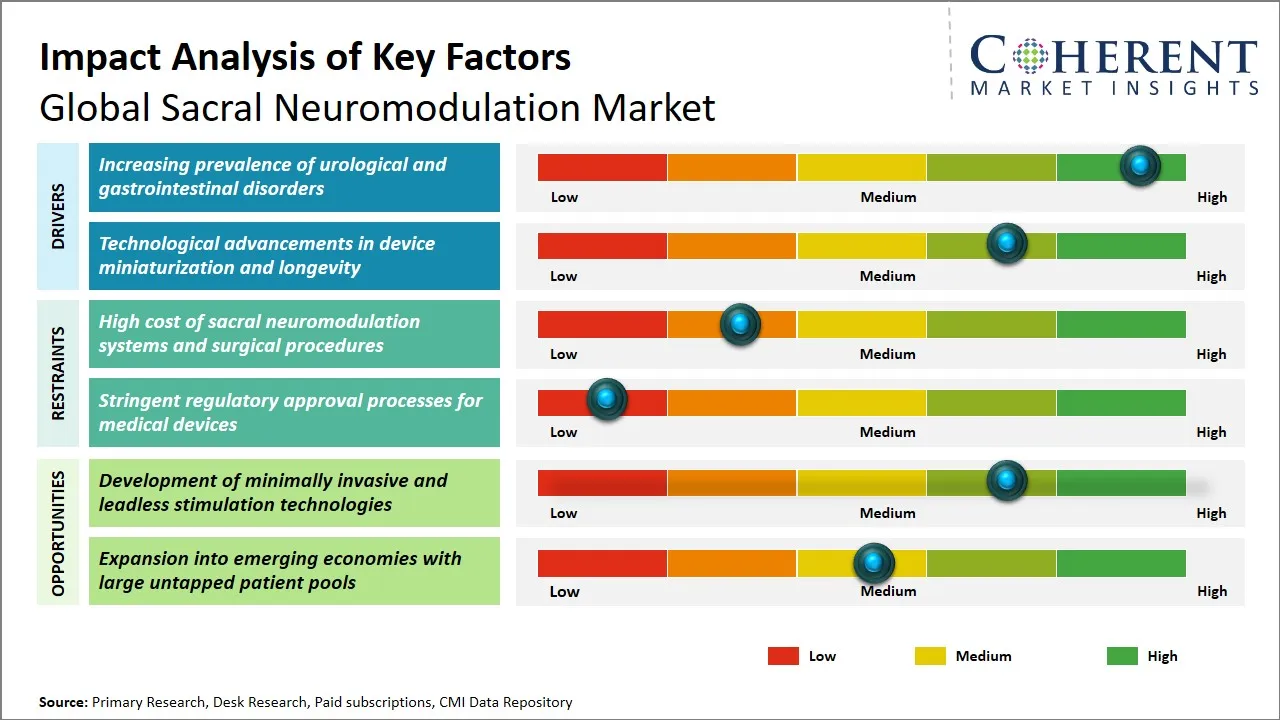
Sacral Neuromodulation Market Driver - Increasing Prevalence of Urological and Gastrointestinal Disorders
One of the main reasons for the rise in acceptance of sacral neuromodulation therapies is the growing prevalence of urological and gastrointestinal disorders such as overactive bladder, fecal incontinence, and chronic constipation. These issues, which have a great impact on the quality of life, are usually very hard to treat with conventional methods, hence, patients and health care professionals are looking for efficient alternatives.
For instance, in February 2025, a study published in Scientific Reports published by Springer Nature Limited examined the global burden of urinary tract infections (UTIs) based on data from the Global Burden of Disease study 2021. The study found that from 1990 to 2021, the number of UTI cases increased by 66.45%, reaching 4.49 billion cases.
A 2025 global gastroenterology study by Elsevier Inc. found that over 40% of adults worldwide suffer from functional gastrointestinal disorders (FGIDs), with women more affected than men. FGIDs lead to lower quality of life and more doctor visits. The prevalence was higher in internet survey respondents (40.3%) compared to household surveys (20.7%), and conditions like irritable bowel syndrome were less common using the Rome IV criteria.
Sacral Neuromodulation Market Opportunity - Development of Minimally Invasive and Leadless Stimulation Technologies
The global sacral neuromodulation market is advancing into an era of technological progress with the introduction of novel leadless and minimally invasive stimulation techniques. In the past, the traditional sacral neuromodulation treatment was associated with surgical implantation of large devices and leads, thereby risking infection, pain and long recovery periods. The introduction of less invasive methods is meant to simplify the procedure and make the patient more comfortable by restricting the volume and frequency of cuts made.
In November 2024, Medtronic, a global leader in healthcare technology, launched the InterStim X Sacral Neuromodulation (SNM) system in India for treating overactive bladder, chronic fecal incontinence, and non-obstructive urinary retention. The minimally invasive therapy targets nerve signals to improve bladder and bowel control in patients who don't respond to other treatments.
Analyst Opinion (Expert Opinion)
- Implantable devices are the main reason for the expansion of the sacral neuromodulation market that offers more efficient ways to treat overactive bladder and the likes. The supporting of regulations in areas such as the U.S. and Europe together with the surge in the demand for minimally invasive procedures are the main growth drivers. Nevertheless, the high treatment costs could be a barrier for the market to enter some regions.
- The key events such as the International Continence Society (ICS) and European Association of Urology (EAU) conferences have played a crucial role in sharing the latest technological developments and overviews of the clinical data. Medtronic’s InterStim systems with their U.S. FDA approvals are a clear indication of the industry’s commitment toward enhancing the treatment availability and patients’ results.
Market Segmentation
- Product Type Insights (Revenue, USD Bn, 2020 - 2032)
- Implantable Pulse Generators (IPGs)
- Lead Electrodes
- External/Non‑Implantable Pulse Generators (EPGs)
- Accessories and System Components
- Application Insights (Revenue, USD Bn, 2020 - 2032)
- Urinary Incontinence (stress, urge, mixed)
- Overactive Bladder (OAB)
- Fecal Incontinence
- Urinary Retention
- Chronic Pelvic Pain and Related Pelvic Floor Disorders
- Others (e.g., gastrointestinal motility disorders, pediatric indications)
- End User Insights (Revenue, USD Bn, 2020 - 2032)
- Hospitals and Large Medical Centres
- Ambulatory Surgical Centers (ASCs)
- Specialty Clinics (Urology/Gastroenterology/Pelvic Health)
- Research and Academic Institutes
- Patient Age Group Insights (Revenue, USD Bn, 2020 - 2032)
- Pediatric
- Adult
- Geriatric
- Technology Insights (Revenue, USD Bn, 2020 - 2032)
- Rechargeable Systems
- Non‑Rechargeable Systems
- Wired/Lead Systems vs Wireless/Leadless Systems
- Trial Systems (temporary leads) vs Permanent Systems
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Medtronic plc
- Boston Scientific Corporation
- Abbott Laboratories
- Axonics Inc
- Nevro Corp
- LivaNova PLC
- NeuroPace Inc
- Synapse Biomedical Inc
- Beijing PINS Medical Co., Ltd
- Saluda Medical Pty Ltd
- Nuvectra Corp
- Johnson and Johnson
- Valencia Technologies
- EBT Medical
- Inspire Medical Systems
Sources
Primary Research Interviews
- Industry Stakeholders
- OEM neuromodulation device manufacturers (senior execs)
- Medical-device component suppliers (R&D heads)
- End Users
- Urology/urogynaecology physicians performing sacral neuromodulation
- Hospital procurement managers for neuromodulation implants
- Regional distributor directors for neuromodulation therapy devices
- Clinical trial coordinators for SNM implants in emerging markets
Government and International Databases
- U.S. Food & Drug Administration (FDA) Medical Device Databases
- European Medicines Agency/EUDAMED
- World Health Organization (WHO) Global Health Observatory or device safety databases
- National health statistics agencies
- National procedure/implant-registry databases
- Public clinical-trial registries
Trade Publications
- Medical Device Network
- Medical Device + Diagnostic Industry (MD+DI) magazine
- Medical Device Business News or DeviceTalks
- Urology Times
- Journal of Medical Devices
- HealthTech Magazine
Academic Journals
- Neuromodulation: Technology at the Neural Interface
- Brain Stimulation
- International Journal of Molecular Sciences
- BJU International
- Journal of Urology
- Autonomic Neuroscience: Basic & Clinical
Reputable Newspapers/Major News Outlets
- The New York Times (health technology section)
- The Guardian (medical-device advances)
- Financial Times (health-tech business coverage)
- Wall Street Journal (device-industry analyses)
- Reuters Health / Reuters Business (medical-device news)
- Bloomberg (medical-technology market coverage)
Industry Associations
- International Neuromodulation Society (INS)
- Neuromodulation Society (India) (TNS)
- Association of British HealthTech Industries (ABHI)
- Society for Brain Mapping and Therapeutics (SBMT)
- American Urological Association (AUA)
- European Association of Urology (EAU)
Public Domain Resources
- NIH NCBI Bookshelf – medical-device therapy overviews
- Open-access clinical-study repositories (PMC)
- Government regulatory guidance documents (FDA, EU)
- National implant-registry summary reports
- WHO technical reports on neurology/device therapy
- Public-domain patent databases (e.g., USPTO)
Proprietary Elements
- CMI Data Analytics Tool: Proprietary analytics tool to analyze real-time market trends, consumer behavior, and technology adoption in market
- Proprietary CMI Existing Repository of Information for Last 8 Years
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients