Chronic pulmonary aspergillosis (CPA) is a serious long-term fungal disease of the lung with a worldwide prevalence. Chronic pulmonary aspergillosis (CPA) complicates conditions including tuberculosis, chronic obstructive pulmonary disease and sarcoidosis, and is associated with high morbidity and mortality. Chronic pulmonary aspergillosis (CPA) is characterized by slowly progressive destruction of lung parenchyma, in the form of single of multiple cavities, nodules, infiltrates or fibrosis, with or without an aspergilloma.
The global chronic aspergillosis treatment market is estimated to be valued at US$ 2,916.3 million in 2021 and is expected to exhibit a CAGR of 4.8 % during the forecast period (2021-2028).
Figure 1.Global Chronic Aspergillosis Treatment Market Share (%) in Terms of Value, By Drug Class, 2021
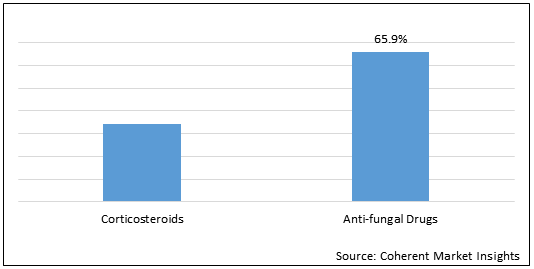
To learn more about this report, Download Free Sample
Increasing incidence and prevalence of aspergillosis is expected to drive the market growth during the forecast period.
Increasing prevalence of aspergillosis is expected to drive the global chronic aspergillosis treatment market growth over the forecast period. For instance, according to the National Organization for Rare Disorders, aspergillosis has been reported from all over the world in recent years. Allergic bronchopulmonary aspergillosis has been estimated to affect about one to four million people worldwide annually. Moreover, according to the same source, chronic pulmonary aspergillosis is estimated to affect about three million people worldwide every year.
Chronic Aspergillosis Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2020 | Market Size in 2021: | US$ 2,916.3 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2021 to 2028 |
| Forecast Period 2021 to 2028 CAGR: | 4.8% | 2028 Value Projection: | US$ 4,056.4 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Astellas Pharma Inc., Abbott Laboratories, Pfizer Inc., Pulmocide Ltd., Johnson & Johnson, Mylan N.V., Glenmark Pharmaceuticals, Merck & Co., Inc., Mayne Pharma Group Limited, GlaxoSmithKline plc., PULMATRiX, Inc., F2G Ltd, Teva Pharmaceutical Industries Ltd., and Novartis International AG |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2.Global Chronic Aspergillosis Treatment Market Share (%), By Disease Type, 2021
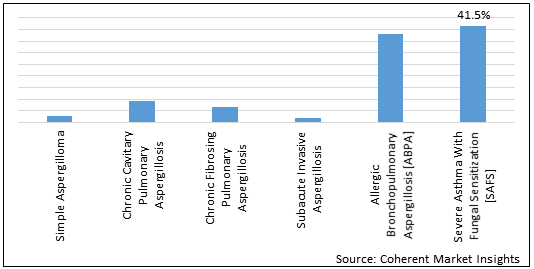
To learn more about this report, Download Free Sample
Robust product pipeline of chronic aspergillosis is expected to drive the market growth during the forecast period.
Robust product pipeline for the treatment of chronic aspergillosis which are expected to launch during the forecast period is anticipated to drive the growth of the global chronic aspergillosis treatment market. For instance, on September 15, 2020, Regeneron Pharmaceuticals, a biopharmaceutical company, initiated phase III, a randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of dupilumab in patients with allergic bronchopulmonary aspergillosis. The study is estimated to be completed by October 23, 2023.
Global Chronic Aspergillosis Treatment Market– Impact of Coronavirus (COVID-19) Pandemic
According to Centers for Disease Control and Prevention, investigators in Spain and the U.S. added 20 new case reports to mounting evidence that patients with coronavirus disease 2019 (COVID-19) are at risk of developing pulmonary aspergillosis. The coronavirus (COVID 19) pandemic and lockdown in various countries across the globe have impacted the financial status of businesses in all sectors. Private healthcare sector is one of the sectors, which is majorly impacted by the COVID-19 pandemic. Moreover, coronavirus pandemic has negatively impacted the development, production, and supply of pharmaceutical products and affected growth of the medication used in treatment of chronic aspergillosis such as antifungal drugs and corticosteroids manufactured by various companies across the regions such as North America, Europe, and Asia Pacific. The supply and production of pharmaceutical products is also affected due to COVID-19 pandemic lockdown imposed globally. This lockdown has resulted in closure of industrial establishments, except manufacturing of essential commodities, and disruption in supply chain of the pharmaceutical products. Thus, COVID-19 pandemic has affected the economy in three main ways; 1) By directly affecting the production and demand; 2) By creating disruptions in distribution channels; and 3) By its financial impact on firms and financial markets. Thus, the impact of coronavirus (COVID-19) pandemic is expected to limit growth of the global chronic aspergillosis treatment market during the forecast period
Global Chronic Aspergillosis Treatment Market: Restraint
The major factors that are expected to hinder growth of the global chronic aspergillosis treatment market include increasing generic competition and complications associated with drugs used in the treatment of chronic aspergillosis. For instance, in November 2016, Ajanta Pharma, a pharmaceutical company, launched voriconazole tablets in 50-mg and 200-mg doses in the U.S. market. These tablets are the equivalent generic version of Vfend, an antifungal drug from Pfizer. Vfend is used to treat conditions such as invasive aspergillosis, candidemia, and serious infections caused by Scedosporium apiospermum and Fusarium species.
Key Players
Major players operating in the global chronic aspergillosis treatment market include Astellas Pharma Inc., Abbott Laboratories, Pfizer Inc., Pulmocide Ltd., Johnson & Johnson, Mylan N.V., Glenmark Pharmaceuticals, Merck & Co., Inc., Mayne Pharma Group Limited, GlaxoSmithKline plc., PULMATRiX, Inc., F2G Ltd, Teva Pharmaceutical Industries Ltd., and Novartis International AG
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients