The percutaneous transluminal coronary angioplasty (PTCA) balloon catheter market is estimated to be valued at USD 2.84 Bn in 2025 and is expected to reach USD 5.48 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.8% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
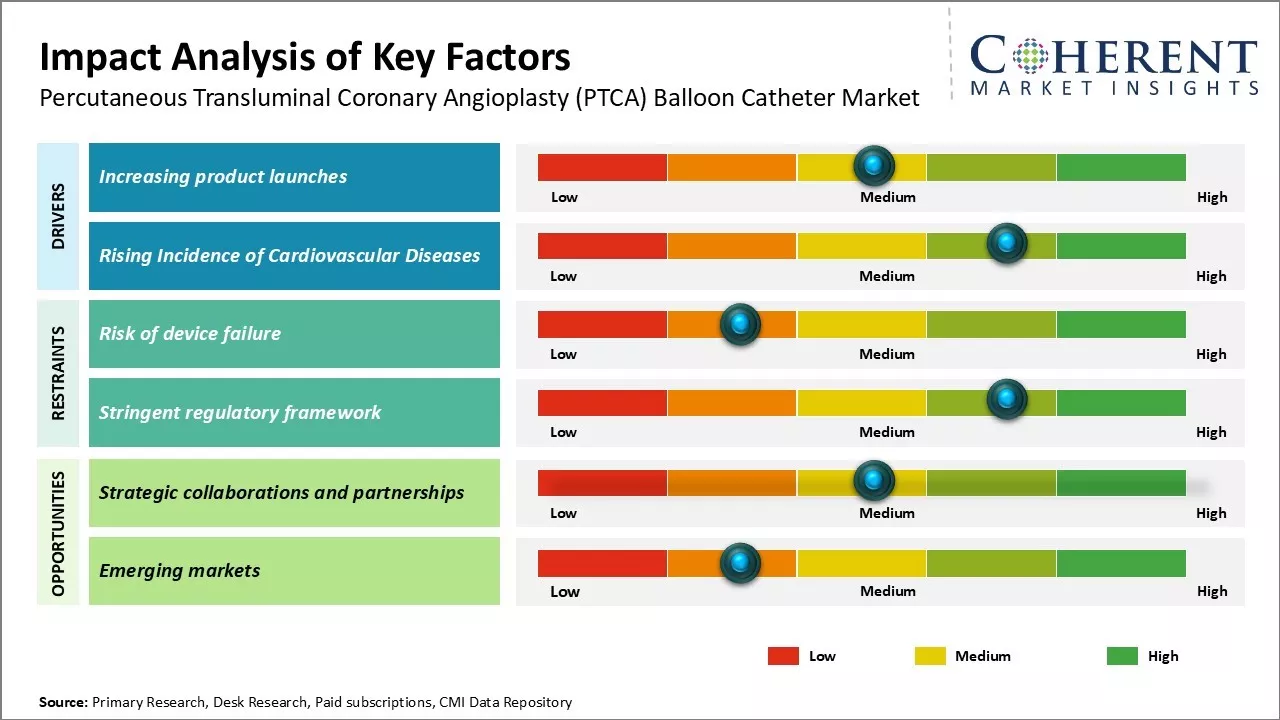
The increasing incidence of cardiac conditions such as acute myocardial infarction and angina is the primary factor driving the growth of the percutaneous transluminal coronary angioplasty (PTCA) balloon catheter market. Additionally, growing preference for minimally invasive procedures over traditional surgery due to advantages such as lesser recovery time, minimal pain, and scarring are also expected to propel the demand for PTCA balloon catheters. However, risks and complications associated with PTCA procedures along with availability of alternative treatment methods such as stents may hamper revenue generation.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
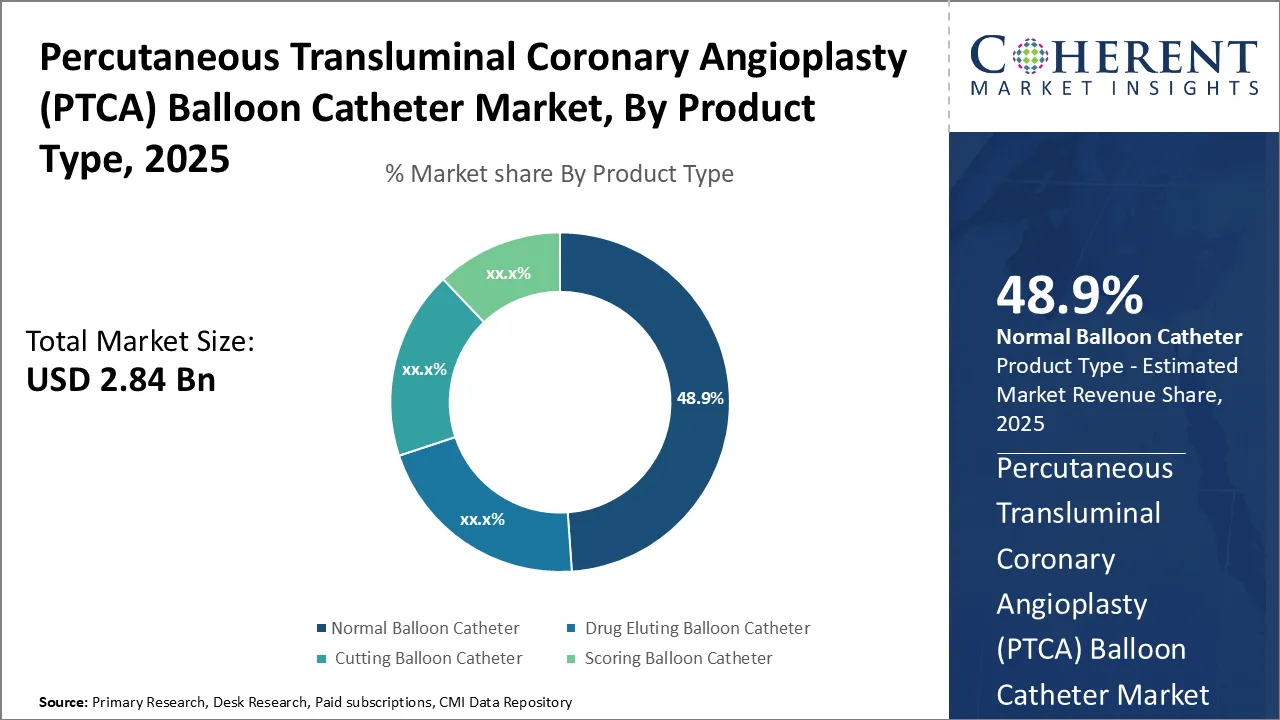
Insights By Product Type - Advanced features drive growth of normal balloon catheter
In terms of product type, normal balloon catheter segment is expected to contribute the highest market share of 48.9% in 2025, owing to its enhanced functionalities compared to other product types. Normal balloon catheters are preferred for their ease of use in majority of regular angioplasty procedures. Featuring high flexibility and trackability, normal balloon catheters can efficiently reach target lesions through tortuous vessels. Their widespread applicability drives their continued dominance as the preferred choice among cardiologists for most PTCA procedures.
Insights By Ballon Material - Material advantages drive preference for POC
In terms of ballon material, polyolefin copolymer (POC) segment is expected to contribute the highest market share of 53.6% in 2025, owing to its unique material properties. POC balloons also demonstrate lower tendency to form cracks or pinholes during inflation-deflation cycles compared to other materials. These material attributes have increased the preference for POC among medical professionals performing complex angioplasty procedures through tortuous vessels. Steady technological advancements to further enhance material properties will drive continued market leadership of POC balloons.
Insights By Application - Prevalence drives PAD segment leadership
In terms of application, peripheral artery disease segment is expected to contribute the highest market share of 65.4% in 2025, owing to its high prevalence. Risk factors for peripheral artery disease include diabetes, high blood pressure, obesity, high cholesterol, smoking, and others. Increased disease awareness campaigns along with aging populations are expected to further drive the growth of PTCA procedures targeting peripheral artery disease.
Need a Different Region or Segment? Download Free Sample
Regional Analysis: Percutaneous Transluminal Coronary Angioplasty (PTCA) Balloon Catheter Market
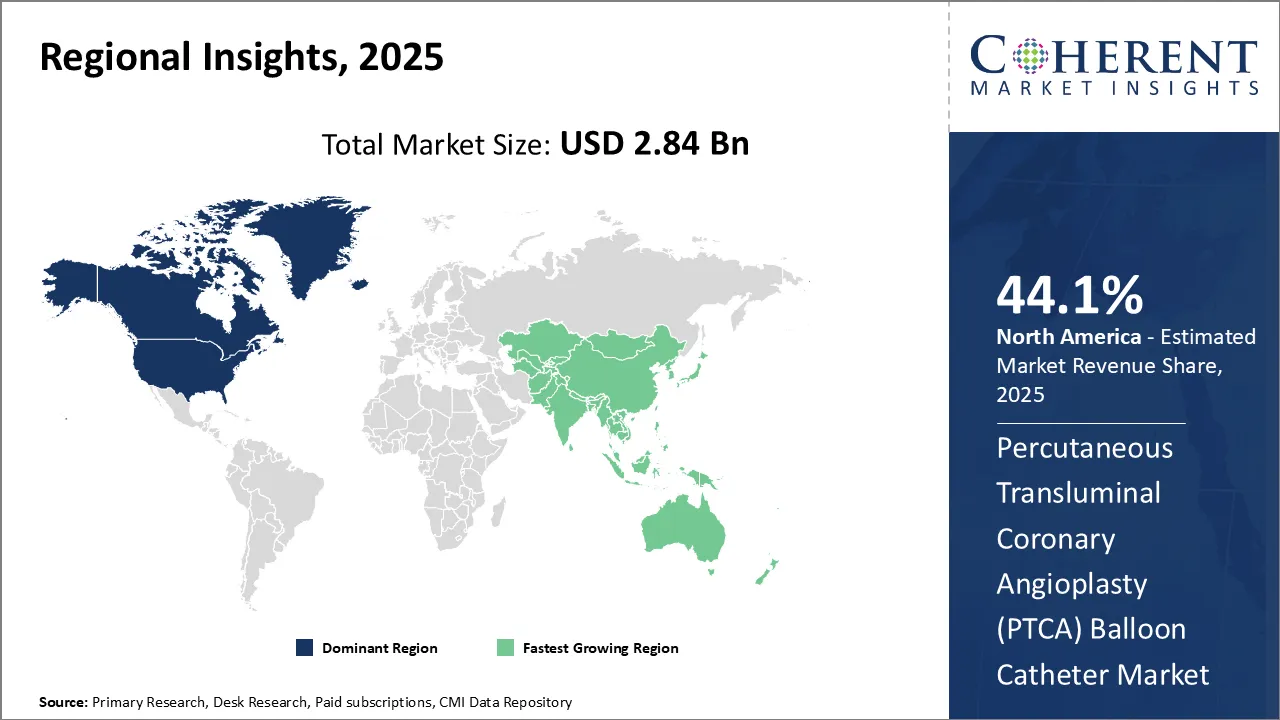
Dominating Region: North America
North America is expected to dominate the percutaneous transluminal coronary angioplasty (PTCA) balloon catheter industry with the highest market share of 44.1% in 2025. This is due to factors such as the strong presence of leading manufacturers, high incidence of cardiovascular diseases, rapidly growing geriatric population, and favorable reimbursement policies.
Fastest-Growing Region: Asia Pacific
Asia Pacific region exhibits the fastest growth with 22.1% market share in 2025 in the percutaneous transluminal coronary angioplasty (PTCA) balloon catheter industry, driven by rising healthcare expenditures, growing medical tourism, and initiatives by governments to improve access to advanced treatment options.
Percutaneous Transluminal Coronary Angioplasty (PTCA) Balloon Catheter Market Outlook for Key Countries
Product innovation in the U.S.
The U.S. percutaneous transluminal coronary angioplasty (PTCA) balloon catheter market is characterized by significant innovation, with manufacturers like Boston Scientific investing heavily in research and development. This focus on novel catheter designs enhances patient outcomes and drives market growth through value-added products.
Advanced technology innovation in Japan
Japan percutaneous transluminal coronary angioplasty (PTCA) balloon catheter market thrives on advanced technology integration, enhancing patient outcomes. Terumo stands out as a key supplier, leveraging its strong local brand presence to deliver innovative and effective catheter solutions tailored to clinical needs.
Health coverage in China
China transluminal coronary angioplasty (PTCA) balloon catheter market is experiencing significant growth, primarily fueled by nationwide reforms aimed at promoting universal health coverage. The improving healthcare infrastructure, making medical services more accessible to the population to enhance access to healthcare services, thereby increasing the demand for advanced medical technologies such as PTCA balloon catheters.
Enhanced access to healthcare in India
India continues to lead the Asia Pacific percutaneous transluminal coronary angioplasty (PTCA) balloon catheter market, driven by a large untreated population and enhanced access to healthcare. Public-private partnerships between hospitals and vendors are improving service delivery, significantly increasing the adoption of advanced medical technologies.
Get actionable strategies to beat competition: Download Free Sample
Top Strategies Followed by Percutaneous Transluminal Coronary Angioplasty (PTCA) Balloon Catheter Market Players
Emerging Startups in the Percutaneous Transluminal Coronary Angioplasty (PTCA) Balloon Catheter Market
Several startups are developing innovative technologies that could transform percutaneous transluminal coronary angioplasty (PTCA) balloon catheter market. Companies like Angiosens are working on smart sensor balloon catheters integrated with AI systems to provide real-time diagnostic data during procedures. Such technologies may optimize outcomes through personalized treatment planning. Heartflow is developing a virtual fractional flow reserve technology that uses CT scans and sophisticated algorithms to digitally simulate a cardiac catheterization. This has the potential to reduce invasive diagnostic tests.
Sustainability is another area attracting startup interest. TeraMedical focuses on producing eco-friendly balloon catheter materials from biomass-derived polymers and biodegradable coatings. Their goals are to replace petroleum-derived plastics and minimize hazardous waste production. In addition, several startups partner with medical product firms and hospitals to pilot test innovative designs and technologies.
Key Takeaways from Analyst
Percutaneous Transluminal Coronary Angioplasty (PTCA) Balloon Catheter Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.84 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.8% | 2032 Value Projection: | USD 5.48 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
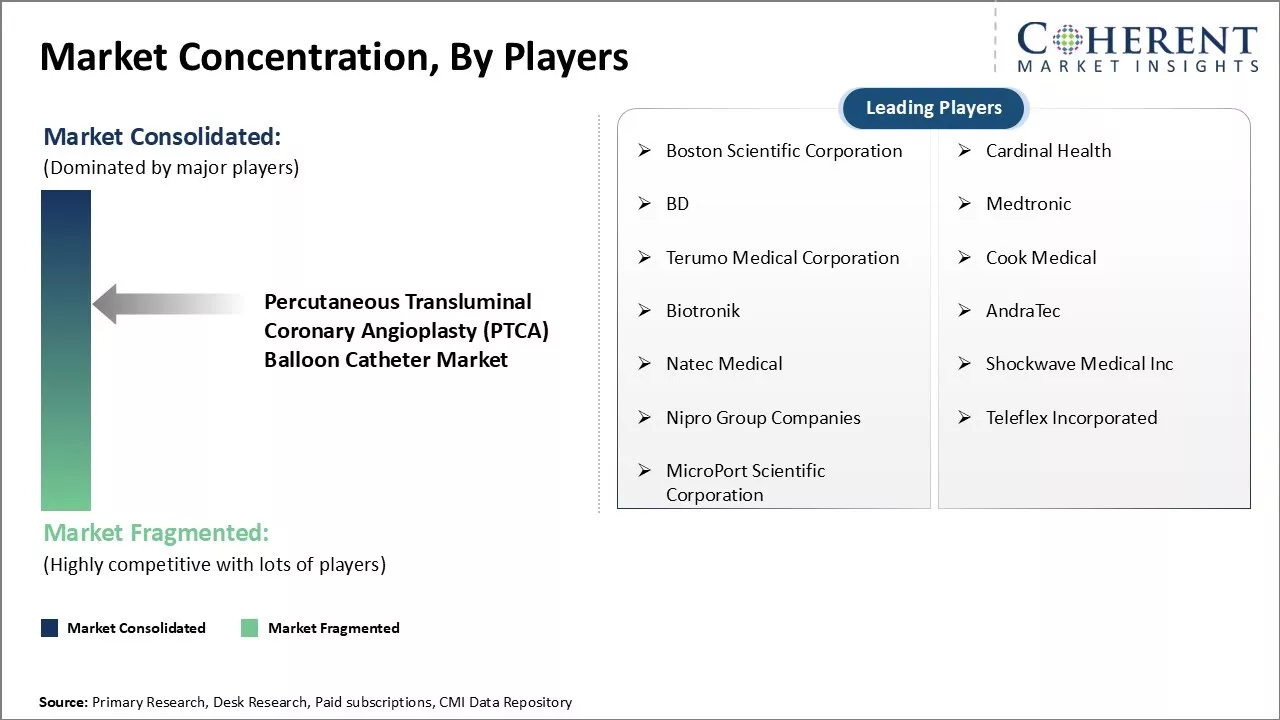
Boston Scientific Corporation, Cardinal Health, BD, Medtronic, Terumo Medical Corporation, Cook Medical, Biotronik, AndraTec, Natec Medical, Shockwave Medical Inc, Nipro Group Companies, Teleflex Incorporated, and MicroPort Scientific Corporation |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Driver - Rising Incidence of Cardiovascular Diseases
The rising prevalence of risk factors such as smoking, obesity, and physical inactivity have led to a global surge in cardiovascular conditions. According to WHO, approximately 17.9 million people died from CVDs in 2019, representing 32% of all global deaths. The majority of these deaths were attributed to heart attacks and strokes. Angioplasty using PTCA balloons is increasingly demanded as a minimally invasive alternative to coronary bypass surgery. Clinical trials highlight its safety, efficacy, and cost-effectiveness in restoring blood flow, making it a preferred option for various coronary syndromes. This growing incidence of cardiac illnesses drives the need for PTCA balloon solutions.
Market Challenge - Risk of Device Failure
One of the major challenges in the percutaneous transluminal coronary angioplasty (PTCA) balloon catheter market is the risk of device failure. Balloon rupture or malfunction during delicate procedures can lead to serious complications, including cardiac damage or even death. This necessitates stringent quality control and extensive clinical testing to minimize risks, as complete elimination of failures is challenging due to high-pressure conditions.
Market Opportunity - Strategic Collaborations and Partnerships
One key opportunity in the percutaneous transluminal coronary angioplasty (PTCA) balloon catheter market is forming strategic collaborations and partnerships. Given the substantial investments required for clinical research and technology advancements, no single manufacturer can achieve success alone. Collaborations enable companies to share resources, expertise, costs, and risks, accelerating the development of safer and more effective products. These synergies will be crucial for gaining a competitive edge, achieving economies of scale, and driving market growth.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients