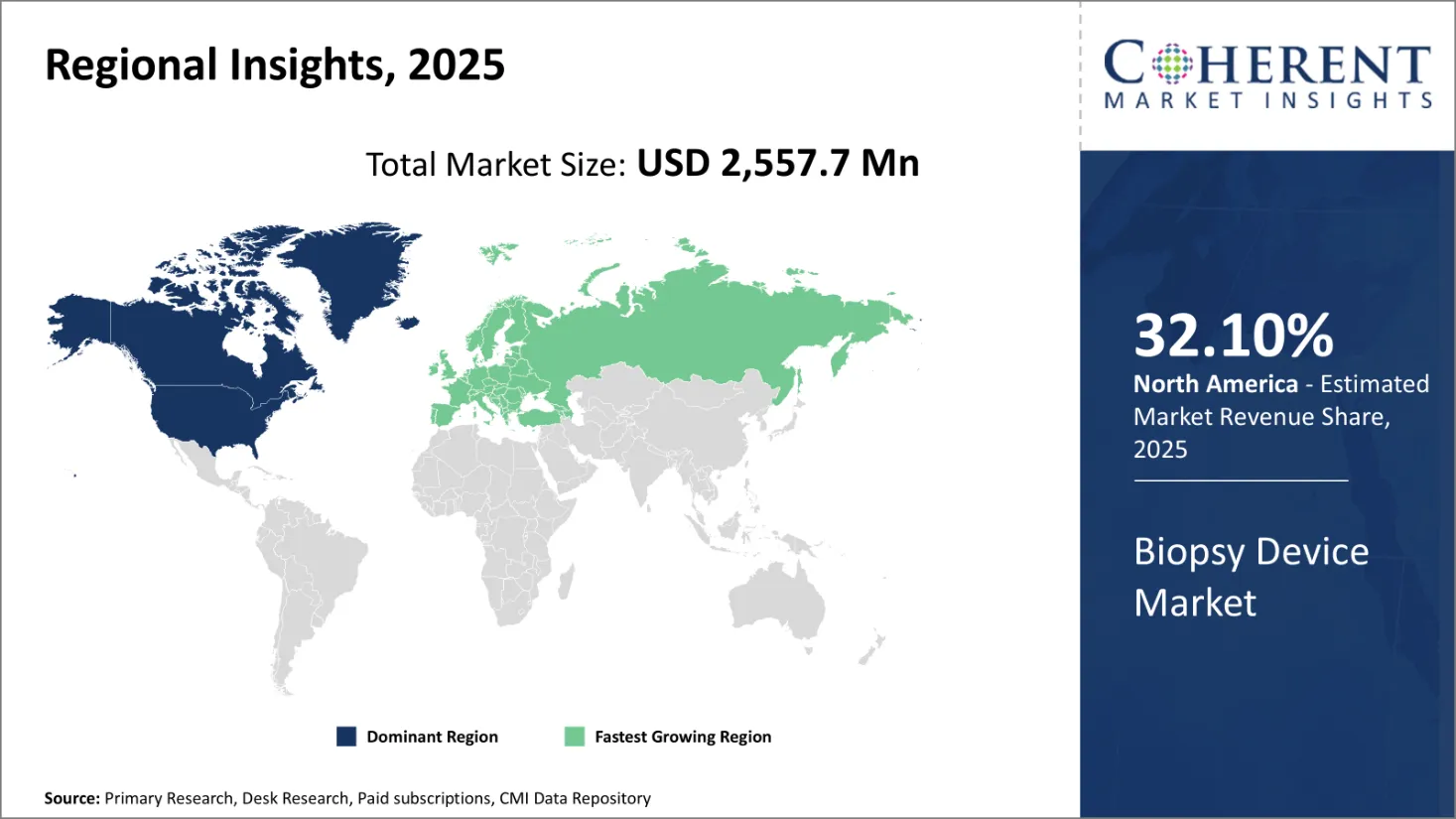
Biopsy Devices Market is estimated to be valued at USD 2,557.7 Bn in 2025 and is expected to reach USD 3,974.6 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 6.5% from 2025 to 2032.
The Biopsy Devices Market Size is expanding rapidly due to the increased need for minimally invasive diagnostics, technical advancements, and the rising incidence of cancer. The needle biopsy segment accounted for the largest market share in 2025 attributed to an increase in the number of cancer cases across the globe.
For instance, in November 2024, Mammotome is excited to unveil the Mammotome AutoCoreTM Single Insertion Core Biopsy System, the first automated spring-loaded core needle device on the market. This innovative launch highlights the company’s unwavering dedication to pioneering breast biopsy technology and improving patient care.
|
Event |
Description and Impact |
|
Shift to single-use biopsy instruments for infection control |
|
|
Rising global cancer incidence |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The biopsy devices market pipeline is dynamic, with ongoing development of new products focused on improving precision, safety, and patient comfort. Innovations include image-guided biopsy devices, single-use instruments for infection control, and integration of AI and robotics to enhance diagnostic accuracy and procedural efficiency. Companies are investing in next-generation devices that offer minimally invasive options and compatibility with advanced imaging modalities, reflecting a strong pipeline of products at various stages of development.
The patent landscape for biopsy devices is characterized by active innovation, with leading manufacturers securing intellectual property for advancements in needle design, guidance systems, and automation technologies. This competitive environment encourages continuous R&D, especially in areas such as vacuum-assisted and core needle biopsy devices, as well as devices that integrate imaging and digital healthcare for improved outcomes.
In terms of biopsy type, the liquid biopsy segment is expected to contribute largest share of the Biopsy Devices market trends in 2025. The liquid biopsy segment within the broader biopsy devices market is experiencing rapid growth, driven by its non-invasive nature, technological advancements, and increasing demand for early cancer detection and monitoring.
Liquid biopsy refers to the analysis of circulating biomarkers such as cell-free DNA (cfDNA), circulating tumor cells (CTCs), and exosomes from bodily fluids like blood or urine, offering a less invasive alternative to traditional tissue biopsies.
In terms of product type, the needle-based biopsy instruments segment is expected to contribute largest share of the Biopsy Devices market trends in 2025. The increasing global prevalence of cancers especially breast, lung, prostate, and liver cancers has significantly driven the demand for needle-based biopsy procedures, which are essential for early and accurate diagnosis.
Innovations such as vacuum-assisted biopsy (VAB) systems and image-guided (ultrasound, CT-guided) procedures have improved sample collection efficiency, accuracy, and patient outcomes. Recent product launches, like Cook Medical's EchoTip AcuCore EUS biopsy needle, highlight ongoing advancements in this segment.
In terms of application, the prostate segment is expected to contribute largest share of the Biopsy Devices market trends in 2025. Prostate biopsy devices are essential for the early detection and diagnosis of prostate cancer, one of the most common malignancies in men. The market is segmented by product type, including needle-based biopsy instruments (such as core biopsy and aspiration devices), vacuum-assisted biopsy devices, and liquid biopsy technologies. Needle-based biopsy instruments remain the most widely used due to their established efficacy and extensive clinical adoption.
In terms of end user, the hospital segment is expected to contribute largest share of the Biopsy Devices market trends in 2025. The hospital segment is the leading end-user in the global biopsy devices market. In 2023, hospitals and breast care centers accounted for the largest share of the market, surpassing other segments such as diagnostic imaging centers and ambulatory surgical centers.
To learn more about this report, Download Free Sample
North America is expected to be the largest market for biopsy devices during the forecast period, accounting for 32.10% of the market share in 2025. The growth of the market in North America is attributed to factors such as the presence of key market players, the launch of novel products, and favorable reimbursement scenarios.
Moreover, inorganic growth strategies such as acquisitions adopted by market players are further anticipated to support the market development over the analysis period.
For instance, on April 27, 2023, announced an all-cash equity transaction in which Quest will acquire Haystack. MRD testing is a rapidly growing category of liquid biopsy tests that detect circulating tumor DNA (ctDNA) in patients' bloodstreams following cancer surgery and treatment. Haystack, founded in 2021, has developed a ctDNA-based technology for MRD detection based on 20 years of research and development.
Europe is expected to be the second-largest market for biopsy devices, accounting for over 23.1% of the market share in 2025. The growth of the market is attributed to the presence of skilled healthcare professionals, top notch healthcare institutes, the growing prevalence of cancer, and the adoption of inorganic growth strategies such as partnerships that are anticipated to support the market development in this region.
For instance, on September 18, 2023, Roche France, and the Institute Gustave Roussyannounced a unique partnership to establish in-house liquid biopsy testing at the Institute Gustave Roussy's facilities in France, by transferring technology from Foundation Medicine's FoundationOneLiquid CDx, a blood-based comprehensive genomic profiling (CGP) test. FoundationOne Liquid CDx analyzes more than 300 cancer-related genes for genomic alterations that cause cancer to grow using a simple blood sample.
The Asia Pacific is expected to be the fastest-growing market for biopsy devices during the forecast period. The growth of the market in Asia Pacific is attributed to growing geriatric population, rise in the healthcare expenditure, and regulatory approvals is expected to support the development of the market over the analysis period.
For instance, on May 30, 2023, Guardant Health, Inc., has approved Guardant360 CDx, a liquid biopsy test for tumor mutation profiling, also known as comprehensive genomic profiling (CGP), in patients with advanced solid cancers. The Guardant360 CDx test has also been approved as a companion diagnostic to identify patients with advanced non-small cell lung cancer (NSCLC) who have epidermal growth factor receptor (EGFR) alterations and may benefit from TAGRISSO (osimertinib) treatment.
The U.S. places significant emphasis on early disease detection and benefits from a high incidence of cancer, which drives demand for advanced biopsy technologies. Additionally, a proactive regulatory environment and strong industry collaborations, along with the presence of key market players and a focus on technological innovation, further reinforce the U.S. as a dominant force in the biopsy devices market.
India represents one of the fastest-growing markets for biopsy devices, propelled by a rising burden of cancer and other chronic diseases, increasing healthcare awareness, and expanding access to healthcare services. The country is witnessing greater adoption of minimally invasive diagnostic procedures and a growing number of diagnostic centers and hospitals equipped with advanced biopsy technologies.
China is a major contributor to the Asia-Pacific biopsy devices market, driven by rapid healthcare modernization, increasing cancer prevalence, and substantial government investment in healthcare infrastructure. The market is benefiting from strong demand for advanced and minimally invasive diagnostic tools, as well as a growing focus on early disease detection.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2,557.7 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.5% | 2032 Value Projection: | USD 3,974.6 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Merit Medical Systems, Inc, Hologic, Inc., Becton, Dickinson and Company, Medtronic plc, Stryker Corporation, Leica Biosystems Nussloch GmbH, Gallini Srl, TSK Laboratory, Remicalm, Meditech Devices, Agilent Technologies Inc., Guardant Health, Inc., Agena Bioscience, nRichDX, Epic Sciences, Inc., Predicine, Inc., Argon Medical Devices, Cook Medical, Hologic Inc., and Intact Medical Corporation |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The increasing prevalence of cancer cases around the world has become a major concern for the healthcare industry and governments. Cancer is one of the leading causes of death globally, and its incidence has been rising steadily over the past few decades.
As per the World Health Organization (WHO) 2022 report, cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020. Breast, lung, colorectal, prostate, and skin were the top five cancer types, accounting for 2.26 Mn, 2.21 Mn, 1.93 Mn, 1.41 Mn, and 1.20 Mn cases worldwide.
Technological advancements in biopsy devices are significantly contributing to the growth of the global biopsy devices market. A biopsy is a medical procedure that involves extracting cell or tissue samples from a patient to examine them under a microscope. Traditionally, biopsy procedures involved invasive surgical methods that caused pain and discomfort for patients.
However, recent technological innovations are enabling minimally invasive biopsy procedures that are faster, safer and more accurate. For instance, in July 2021, GE Healthcare, a multinational medical technology company, announced the installation of Serena Bright in five hospitals and radiology centers across the U.S. Serena Bright is the industry's first contrast-guided biopsy solution, assisting clinicians and patients in their fight against breast cancer.
The technology, which received 510(k) clearance from the U.S. FDA in May 2020, allows patients to have their breast biopsy exams with contrast guidance using the same mammography equipment, in the same room, and with the same staff as the screening or diagnostic mammogram.
The increased government and private funding for cancer research has significantly boosted the biopsy devices market. Governments across the world are allocating higher budgets for cancer research and diagnostic technologies in view of rising cancer cases.
For instance, on November 13, 2023, the American Cancer Society (ACS), the U.S. largest non-government, non-profit funder of cancer research, approved funding for 131 new Extramural Discovery Science (EDS) research and career development grants totaling US$64.5 million. Beginning in 2024, the grants will support researchers at 72 institutions across the U.S.
The adoption of robot-assisted biopsy procedures is having a major influence on the biopsy devices market. With the introduction of robotic technology, biopsies can now be performed with increased precision, safety and accuracy. Robotic systems allow for minimally invasive procedures with 3D imaging and small instrument movements. This translates to reduced risk of complications for patients and shorter recovery times.
For instance, in May 2022, UC Davis Health, one of America's leading cancer treatment hospitals, announced that its physicians performed the first single-anaesthesia diagnosis and treatment of lung cancer in the UC Health System using a fully robotic approach. The robotic-assisted bronchoscopy approach enables the diagnosis and removal of a lung cancer mass in a single surgery. It reduces patients' anxiety and unnecessary waiting time.
Liquid biopsy trend has significantly influenced the biopsy devices market in recent years. With advancements in genomics and precision medicine, there is a growing preference for minimally invasive procedures over surgical biopsies. Liquid biopsy is an emerging technology that analyzes blood samples to detect cancer by finding DNA from tumor cells that are circulating in the bloodstream. This provides valuable information about any genetic mutations occurring in the cancer and helps doctors choose the most effective targeted therapies.
Moreover, the adoption of organic growth strategies, such as new products launched adopted by market players, is anticipated to further bolster the market development. For instance, on November 1, 2023, Illumina Inc., a leader in DNA sequencing and array-based technologies, announced the launch of a new generation of its distributed liquid biopsy assay for genomic profiling. The TruSight Oncology 500 ctDNA v2 (TSO 500 ctDNA v2) research assay offers noninvasive comprehensive genomic profiling (CGP) of circulating tumor DNA (ctDNA) from blood when tissue testing is unavailable or to complement tissue-based testing.
The biopsy devices market has been witnessing a steady shift towards vacuum-assisted biopsy in recent times owing to its significant clinical benefits over traditional core needle biopsy procedures. Vacuum-assisted biopsies have emerged as a more accurate alternative for sampling breast lesions as they provide higher tissue sample quality and quantity for pathological examination. This helps achieve superior diagnostic accuracy, especially in differentiating between benign and malignant tumors, as compared to core needle biopsies.
The emerging economies in the Asia Pacific and Latin America regions provide huge growth potential for the biopsy devices market. These developing nations are witnessing improvements in healthcare access and infrastructure, backed by strong economic growth over the past decades. Countries like India, China, Brazil, and Mexico have invested heavily to strengthen primary care and introduce universal healthcare programs. This is increasing patients' ability to seek medical help and diagnostic procedures.
For instance, in September 2022, AstraZeneca India, a multinational pharmaceutical and biotechnology company, collaborated with the Cancer Awareness Prevention and Early Detection (CAPED) Trust, a grass root non-profit working in healthcare since 2014, to expand its flagship initiative 'Ganga Godavari Cancer Screening Program' in Mathura, India. The event was held at the RKMSC Hospital in Vrindavan, India.
The program, which is supported by the Charities Aid Foundation (CAF) India, aims to raise cancer awareness and detect oral, breast, and cervical cancers in their early stages among women through specialized screening camps.
The introduction of innovative biopsy gun devices could provide promising growth prospects for the biopsy devices market. Biopsy gun devices allow for a less invasive tissue collection method compared to traditional biopsy needles. They utilize spring-powered technology to effectively obtain tissue samples from within the body, reducing patient discomfort and procedure time.
For instance, in August 2022, Mammotome, a leader in breast care, announced the launch of the Mammotome DualCore biopsy system, the company's first dual stage core biopsy instrument. This new core biopsy device enhances the Mammotome ultrasound-guided breast biopsy portfolio, which also includes the Mammotome Elite Tetherless Vacuum-Assisted Biopsy Device (tetherless VABB) and the Mammotome Revolve Dual Vacuum-Assisted Breast Biopsy System (tethered VABB).
The development of minimally invasive biopsy techniques could provide a huge opportunity for growth in the biopsy devices market. Minimally invasive procedures are less traumatic for patients as they involve smaller incisions and cause less damage to tissue compared to traditional open biopsy methods.
This allows for shorter procedure times, less pain and discomfort, lower risks of complications, and shorter recovery periods. As healthcare increasingly focuses on providing less invasive options that improve patient outcomes and experiences, the demand for minimally invasive tools and techniques across medical specialties is anticipated to increase over the forecasted period.
*Definition: A biopsy is a surgical process that involves the removal of cells, tissue, or fluid for analysis by a medical pathologist. Biopsies are performed by healthcare professionals when areas of concern are identified or when an individual exhibits symptoms or signs of certain disorders, such as cancer, etc. The devices that are utilized to carry out these procedures are called biopsy devices.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients