Global Eliquis Market Size and Forecast – 2025 to 2032
The global Eliquis market is estimated to be valued at USD 7.90 Bn in 2025 and is expected to reach USD 11.30 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 5.2% from 2025 to 2032. Continued innovation in drug delivery and greater adoption in emerging markets are key trends shaping the market's future trajectory.
Key Takeaways of the Global Eliquis Market
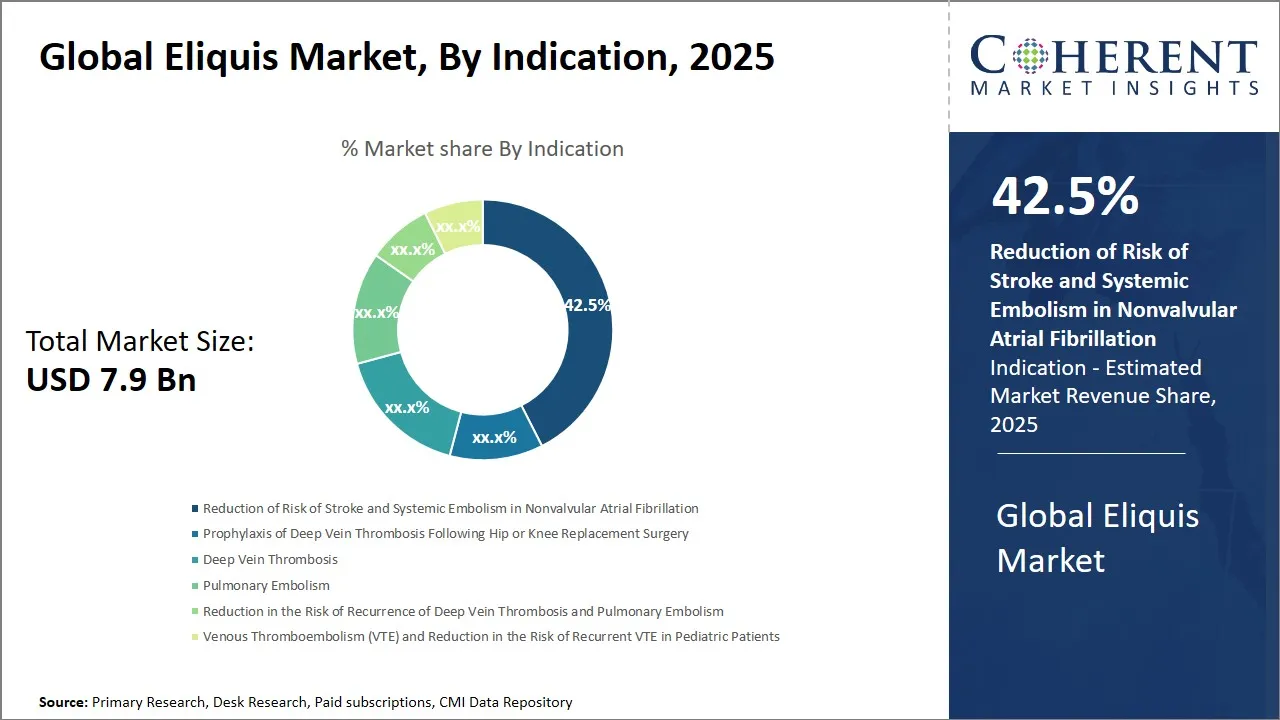
- Reduction of risk of stroke and systemic embolism in nonvalvular atrial fibrillation holds the largest market share by indication, accounting for an expected 42.5% in 2025.
- In terms of strength, the 5 mg segment is expected to lead the market with a 64.2%share in 2025.
- As for dosage form, the oral tablet segment is expected to dominate, contributing 67.1% to the global Eliquis market in 2025.
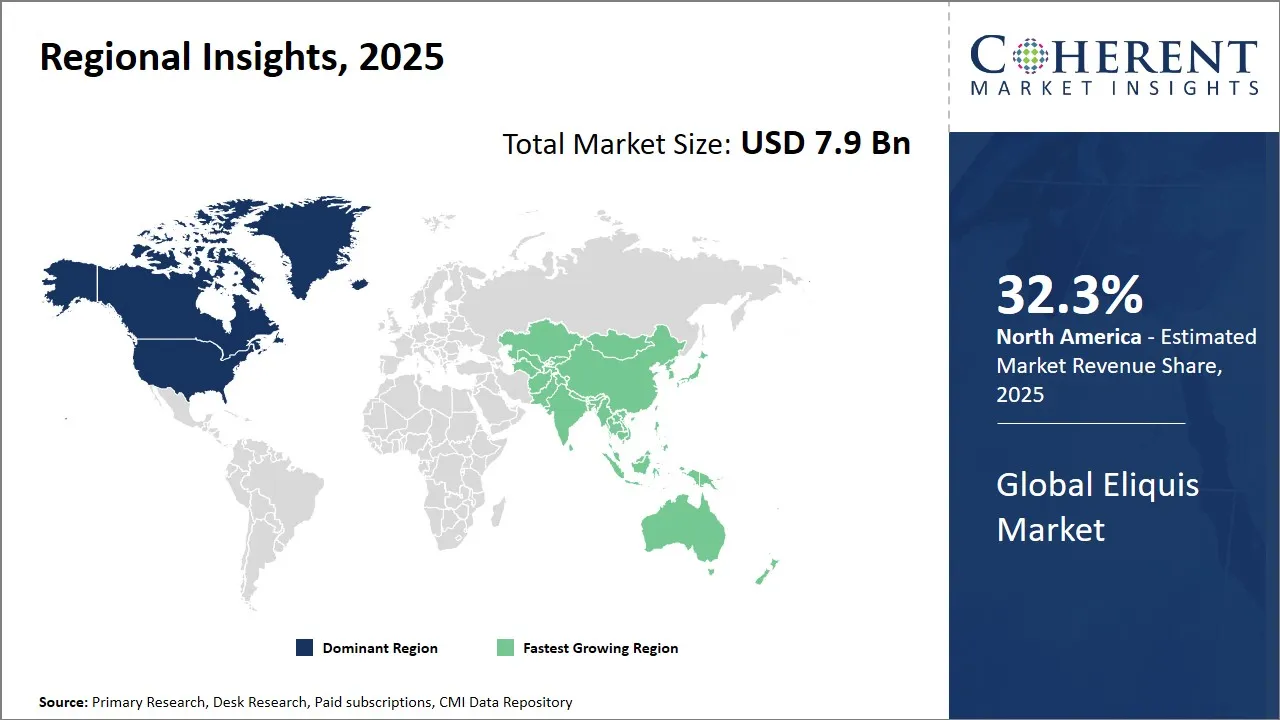
- North America is expected to lead the market, holding a share of 32.3% in 2025. Asia Pacific is anticipated to be the fastest-growing region, with a market share of 25.5% in 2025.
Market Overview
The global Eliquis market has experienced significant growth in recent years, driven by the increasing prevalence of cardiovascular diseases, such as atrial fibrillation (AFib) and venous thromboembolism (VTE), which require long-term anticoagulation therapy. Eliquis, developed by Bristol-Myers Squibb and Pfizer, is a leading direct oral anticoagulant (DOAC) known for its efficacy and safety profile compared to traditional warfarin. The market has benefited from the greater awareness of stroke prevention and favorable clinical guidelines recommending DOACs over older therapies. North America and Europe dominate the market due to high adoption rates, robust healthcare infrastructure, and strong reimbursement policies, while emerging economies in Asia Pacific and Latin America are expected to witness accelerated growth due to improving healthcare access and increasing diagnosis rates.
Current Events and Its Impact
|
Current Events |
Description and its impact |
|
U.S. Government's "Maximum Fair Price" for Eliquis Under Inflation Reduction Act |
|
|
Eliquis Secures CHMP Nod for Pediatric VTE Indication and New Child-Friendly Formulations |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Eliquis Market Insights, By Indication - Reduction of Risk of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation Leads due to Its High Prevalence and the Effectiveness of Eliquis in Managing this cardiovascular disease
In terms of indication, the reduction of risk of stroke and systemic embolism in nonvalvular atrial fibrillation segment is expected to command 42.5% of share of the global Eliquis market in 2025. This dominance is fundamentally driven by the growing prevalence of atrial fibrillation worldwide, particularly in aging populations, which increases susceptibility to strokes and systemic embolic events. Eliquis (apixaban), as a direct oral anticoagulant (DOAC), has established a strong clinical reputation for effectively reducing these risks without the need for routine blood monitoring, which is a key advantage over traditional therapies such as warfarin.
According to the data provided by The Lancet Regional Health Journal of Europe in February 2024, Atrial fibrillation (AF) is the most common cardiac arrhythmia, affecting 1.5-2% of European adults, with an anticipated surge to 9.5% in individuals aged over 65 by 2060. As a primary contributor to stroke, dementia, heart failure, and premature mortality, AF constitutes a significant threat to the expanding elderly demographic and healthcare systems.
Clinicians prefer Eliquis in nonvalvular atrial fibrillation (NVAF) due to its balanced risk–benefit profile providing significant stroke prevention while demonstrating a lower incidence of major bleeding events. This safety aspect enhances patient adherence and broadens the eligible patient population.
Eliquis Market Insights, By Strength – 5 mg Strength Dominates Eliquis Market Due to Standard Adult Dosing and Broad Indication Coverage
The 5 mg strength of Eliquis is expected to command 64.2% market share for the year 2025 primarily because it serves as the standard dosing regimen for the majority of adult patients across its most common indications. In the prevention of stroke and systemic embolism in adults with non-valvular atrial fibrillation (NVAF), the recommended dose is typically 5 mg taken twice daily. This indication alone accounts for a substantial portion of Eliquis prescriptions globally, given the high prevalence of NVAF among aging populations. Furthermore, the 5 mg dose is also widely used in the treatment and secondary prevention of deep vein thrombosis (DVT) and pulmonary embolism (PE), further amplifying its usage across both acute and long-term care settings.
Another factor driving the dominance of the 5 mg strength is its clinical versatility and familiarity among prescribers. Physicians are more inclined to prescribe standard-dose formulations that are well-supported by robust clinical trial data, such as the ARISTOTLE and AMPLIFY trials, which validated the efficacy and safety of the 5 mg dose in adult populations. Additionally, its fixed-dose oral administration without the need for routine coagulation monitoring makes it preferable over traditional anticoagulants, especially in outpatient and chronic management scenarios. As a result, the 5 mg strength has become the go-to option for a large portion of the adult population, securing its position as the market leader within the Eliquis product line.
Eliquis Market Insights, By Age Group – Adult Age Group Leads Eliquis Market Share Due to High Disease Burden and Broad Indication Scope
The adult segment is estimated to hold 53.9% share in the Eliquis market for the year 2025 primarily due to the high prevalence of conditions it is approved to treat—particularly non-valvular atrial fibrillation (NVAF), deep vein thrombosis (DVT), and pulmonary embolism (PE)—which are significantly more common in adults, especially those aged 65 and above. These conditions are linked with age-related risk factors such as hypertension, diabetes, heart failure, and history of stroke or transient ischemic attack (TIA), making anticoagulation therapy essential in a large segment of the adult population. Eliquis' favorable safety profile, particularly its lower risk of major bleeding compared to traditional anticoagulants like warfarin, further supports its widespread use among adult patients with comorbidities.
Stroke incidence is substantially higher in adults compared to children, underscoring the larger therapeutic need in the adult population. According to global estimates, provided by The Lancet Journal, one in four adults over the age of 25 will experience a stroke during their lifetime, reflecting the significant burden of disease among aging populations. In contrast, strokes in children are considerably rarer, with the World Stroke Organization reporting an incidence of approximately 25 per 100,000 newborns and 12 per 100,000 children under 18 years of age.
Data from the Children’s Hospital of Philadelphia further highlights this disparity, noting that nearly 800,000 adults in the U.S. alone suffer a stroke each year. This overwhelming difference in prevalence emphasizes why the adult population remains the primary focus for stroke prevention therapies like Eliquis. Its widespread use in managing stroke risk associated with non-valvular atrial fibrillation and other cardiovascular conditions further supports its dominance in the adult anticoagulant market.
Regional Insights
To learn more about this report, Download Free Sample
North America Eliquis Market Analysis and Trends
The North America Is projected to hold the largest share of 32.3% in 2025 in the global Eliquis market. In North America, the dominance is driven by well-established healthcare infrastructure, strong pharmaceutical industry presence, and favorable government policies supporting innovation in anticoagulant therapies. The U.S. and Canada have advanced reimbursement frameworks and a robust patient base managing cardiovascular diseases, which fuels consistent demand for Eliquis. Additionally, North America houses headquarters and major R&D centers of key players such as Bristol-Myers Squibb and Pfizer, which co-market Eliquis. The region benefits from high physician awareness and widespread insurance coverage, supporting strong prescription volumes. Trade dynamics favor efficient distribution networks and regulatory pathways that expedite access to novel drugs like Eliquis. In addition, collaborations between pharmaceutical giants and academic institutions in North America bolster research and development efforts for Eliquis, enhancing therapeutic advancements. The region's proactive approach to healthcare innovation ensures continuous adaptation to emerging medical needs, sustaining Eliquis' market leadership. Moreover, patient advocacy groups play a crucial role in raising awareness about stroke prevention and anticoagulation therapy, further supporting Eliquis' widespread adoption and market growth in North America.
Asia Pacific Eliquis Market Analysis and Trends
The Asia Pacific region is expected to exhibit the fastest growth in the Eliquis market due to the rising prevalence of atrial fibrillation and venous thromboembolism, growing healthcare expenditure, and improving access to advanced healthcare services. Government initiatives to elevate healthcare standards, enhance insurance penetration, and promote chronic disease management have enabled broader patient accessibility. A consensus document from the Asian Pacific Society of Cardiology (2021) highlights that atrial fibrillation (AF) imposes a disproportionately high disease burden in Asia‑Pacific, projected to affect roughly 72 million people by 2050, and emphasizes that direct oral anticoagulants (DOACs) like apixaban should be preferred over vitamin K antagonists given their superior balance of stroke prevention and bleeding risk in this population. Meta-analyses of major randomized controlled trials (including ARISTOTLE) confirm that apixaban reduces intracranial hemorrhage and major bleeding compared to warfarin in Asian patients treated for AF and venous thromboembolism. This strong clinical validation is driving faster adoption of Eliquis among physicians across China, Japan, India, South Korea, and Southeast Asia.
Global Eliquis Market Outlook for Key Countries
U.S. Eliquis Market Analysis and Trends
The U.S. Eliquis market is characterized by high adoption rates of Eliquis, driven by extensive clinical evidence supporting its efficacy and safety in preventing stroke and treating thromboembolic disorders. Bristol-Myers Squibb and Pfizer play pivotal roles through comprehensive marketing and education campaigns targeted at cardiologists and general practitioners. The country’s advanced regulatory environment and reimbursement systems facilitate timely drug availability and encourage innovation. Additionally, U.S.-based research initiatives continue to build on Eliquis’s clinical applications, reinforcing its leadership position in the anticoagulant space. Moreover, in the U.S., Eliquis (apixaban), a drug jointly developed and commercialized by Pfizer and Bristol Myers Squibb, will have its price negotiated under the Medicare Drug Price Negotiation Program established by the Inflation Reduction Act (IRA). The "Maximum Fair Price" (MFP) for a 30-day supply of Eliquis, as determined by the U.S. Department of Health and Human Services (HHS), will be USD 231, effective January 1, 2026. This price is for the U.S. Medicare market.
Germany Eliquis Market Analysis and Trends
A 2018 population-based modeling study estimated the cumulative impact of transitioning German NVAF patients from vitamin K antagonists (VKAs) to apixaban between 2017 and 2030. The model projected that such a shift could prevent approximately 52,185 major clinical events, including 15,383 non-fatal strokes/systemic embolisms, 22,483 major bleeds, and 14,319 deaths, resulting in an estimated 109,887 life-years gained. This substantial projected benefit demonstrates both individual health gains and public health value, reinforcing German guidelines and prescribing practices that favor NOACs—especially apixaban—for stroke prevention in atrial fibrillation. These real-world data and population-level models together highlight the strong clinical outcomes and potential long-term societal benefits of widespread apixaban use in Germany’s NVAF population.
China Eliquis Market Analysis and Trends
A Hong Kong–based economic model evaluated apixaban versus warfarin in 40,569 NVAF patients, projecting that apixaban would prevent more thromboembolic and bleeding events. The resulting incremental cost-effectiveness ratio (ICER) was estimated at USD 7,057 per QALY—well below China’s 2014 GDP per capita threshold of USD 33,534, translating to a 96–98% probability of being cost-effective. Additionally, a 2019 network meta-analysis of Asian cohorts (including mainland China) found apixaban demonstrated significant reductions in ischemic stroke (HR 0.70), all-cause mortality (HR 0.66), and major bleeding (HR 0.58) when compared to warfarin. These findings reinforce that apixaban is not only clinically advantageous but also economically and socially favorable for NVAF treatment in China.
Japan Eliquis Market Analysis and Trends
Japan continues to lead in terms of clinical adoption of novel anticoagulants, leveraging an aging population with high cardiovascular disease burden. The government’s stringent drug approval and reimbursement policies ensure that only clinically effective therapies like Eliquis gain market traction. Pfizer and Bristol-Myers Squibb actively engage with key opinion leaders and medical societies to maintain Eliquis’s leading position. Japan’s strong emphasis on evidence-based medicine and post-marketing surveillance adds to clinician confidence and patient acceptance. The country’s advanced healthcare infrastructure supports extensive outpatient and inpatient use of Eliquis.
India Eliquis Market Analysis and Trends
India’s market shows rapid expansion driven by increasing awareness about cardiovascular health and rising prevalence of atrial fibrillation and venous thromboembolism. While challenges such as cost sensitivity and healthcare access remain, government programs aimed at improving insurance coverage and healthcare infrastructure are improving patient reach. Pfizer and Bristol-Myers Squibb focus on physician education and partnerships with private and public sector hospitals to boost adoption. Moreover, In India, Eliquis (apixaban) is a significant medication for reducing stroke risk in patients with nonvalvular atrial fibrillation and for treating and preventing venous thromboembolism. AstraZeneca India in January 2024 announced the approval of Andexanet Alfa, a drug that can reverse the effects of apixaban in emergency situations involving life-threatening bleeding. Additionally, Indian pharmaceutical companies are involved in the production and supply of apixaban active pharmaceutical ingredient (API). The approval of Andexanet Alfa enhances physician confidence in prescribing Eliquis by offering a reliable reversal agent for emergencies, thereby supporting safer use. Additionally, local API production by Indian firms ensures cost-effective supply and wider market penetration of apixaban across India.
Pricing Analysis of Eliquis
- Eliquis (apixaban), co-marketed by Bristol-Myers Squibb (BMS) and Pfizer, exhibits significant price variation across global markets. In the U.S., Eliquis is priced at USD 657.23 for a pack of 60 tablets (both 2.5 mg and 5 mg), reflecting its premium positioning as a leading non-vitamin K oral anticoagulant (NOAC). In contrast, Canada offers the same pack size at USD 189.99, demonstrating lower pricing due to government negotiations. The United Kingdom lists the 5 mg strength at USD 209.99 for 112 tablets, while Australia has a higher price at USD 487.99 for 180 tablets, likely due to different reimbursement structures. Emerging markets like India show competitive pricing, with USD 255.99 for 120 tablets (5 mg), making it more accessible.
- In Europe, Germany maintains a moderate price of USD 100.148 for 60 tablets (both strengths), whereas Greece has a notably lower price for the 2.5 mg strength at USD 34.608 for 20 tablets. The United Arab Emirates (UAE) and Romania price Eliquis at USD 83.85 and USD 67.50 for 60 tablets, respectively, indicating regional affordability strategies. Turkey offers one of the lowest prices at USD 99.99 for 56 tablets (5 mg), while Malaysia further reduces costs to USD 48.43 for 30 tablets (2.5 mg). France stands out with the lowest price at USD 14.05 for 60 tablets (2.5 mg), likely due to strict price controls. Mexico ranges between USD 105- USD 150 for 60 tablets (5 mg), aligning with middle-income market trends.
- These variations highlight BMS and Pfizer’s market-specific pricing strategies, balancing affordability with revenue optimization, particularly in markets with strong generic competition or stringent healthcare regulations. The U.S. remains the highest-priced market, while Europe and Asia-Pacific regions benefit from lower costs due to centralized procurement and regulatory controls.
Market Players, Key Developments, and Competitive Intelligence
To learn more about this report, Download Free Sample
Key Developments
- In December 2024, according to National Health Service UK, approximately one hundred people each month in England have been spared from a stroke due to the National Health Service (NHS) lifesaving rollout of blood-thinning medication. The NHS prevented 1,200 strokes by prescribing blood-thinning medication to one million people with Atrial Fibrillation (AF). This intervention reduced stroke risk by two-thirds and helped prevent 100 strokes per month. The NHS also improved AF detection and launched a campaign urging quick action at stroke symptoms. The U.K. government is focused on reducing cardiovascular deaths through better detection of high blood pressure and increased use of lipid-lowering therapies.
- In March 2024, as per data published by the National Library of Medicine, The Chinese Society of Cardiology and the Heart Rhythm Committee have jointly developed the Chinese Guidelines for the Diagnosis and Management of Atrial Fibrillation, which propose a new stroke risk score (CHA2DS2-VASc-60) for the Asian population, emphasize the importance of early rhythm control, and highlight catheter ablation's central role in managing AF. These guidelines aim to improve AF management and the prognosis of patients through the timely application of new technologies and concepts.
Market Report Scope
Eliquis Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 7.90 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.2% | 2032 Value Projection: | USD 11.30 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer Inc. and Bristol-Myers Squibb Company |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Eliquis Market Dynamics
To learn more about this report, Download Free Sample
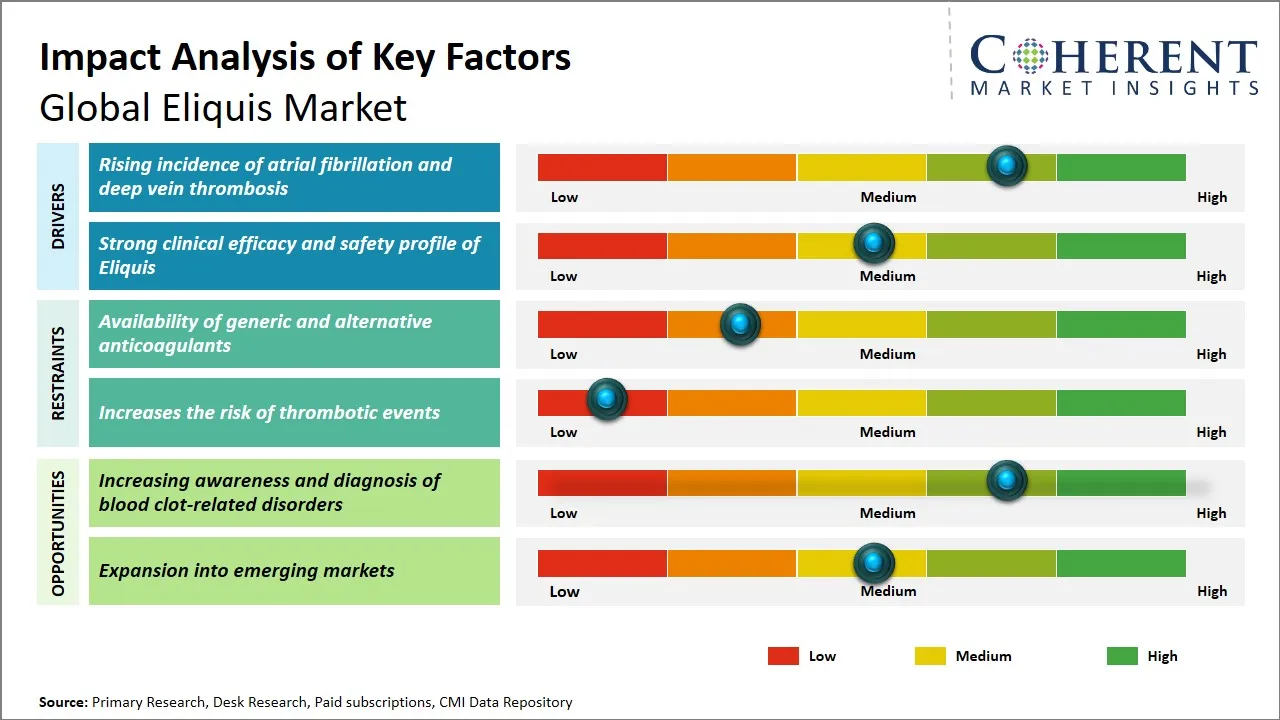
Market Driver - Rising incidence of atrial fibrillation and deep vein thrombosis
The increasing prevalence of atrial fibrillation (AF) and deep vein thrombosis (DVT) is significantly driving the demand for Eliquis globally. As AF is one of the most common cardiac arrhythmias, it elevates the risk of stroke, necessitating effective anticoagulant therapies to manage and reduce thromboembolic events. Similarly, DVT, a condition characterized by blood clots in deep veins, requires reliable anticoagulation to prevent complications such as pulmonary embolism. Eliquis, being a novel oral anticoagulant with proven efficacy and a favorable safety profile compared to traditional therapies, is becoming a preferred choice among healthcare providers. The aging population, lifestyle changes, and increased detection rates contribute to the rising incidence of these conditions, thereby expanding the pool of patients eligible for anticoagulant treatment. This trend is prompting physicians to increasingly prescribe Eliquis, further reinforcing its position in the global anticoagulant pharmaceutical landscape.
Market Challenge - Increasing awareness and diagnosis of blood clot-related disorders
The global Eliquis market presents a significant growth opportunity driven by the increasing awareness and diagnosis of blood clot-related disorders. As healthcare systems worldwide enhance patient education and screening protocols, more individuals are being diagnosed with conditions such as deep vein thrombosis (DVT), pulmonary embolism (PE), and atrial fibrillation, which require effective anticoagulant therapy. Public health campaigns, improved diagnostic technologies, and expanding access to healthcare, especially in emerging economies, contribute to earlier detection rates. This rising diagnosis rate directly fuels demand for innovative and effective anticoagulants like Eliquis, which is favored for its efficacy, safety profile, and ease of use compared to traditional therapies such as warfarin. Additionally, growing awareness among healthcare professionals about the benefits of novel oral anticoagulants (NOACs) is encouraging their adoption as first-line treatments. This trend is further supported by increasing investments in healthcare infrastructure and training programs aimed at improving diagnostic accuracy and patient management. Consequently, Eliquis manufacturers can capitalize on this expanding pool of diagnosed patients, leveraging educational initiatives and evidence-based marketing to strengthen market penetration. The ongoing emphasis on early and accurate diagnosis thus creates an expanding therapeutic window, positioning Eliquis as a preferred option in managing blood clot-related disorders globally.
Analyst Opinion (Expert Opinion)
- The global Eliquis market continues to demonstrate robust growth, driven by rising prevalence of atrial fibrillation (AFib) and venous thromboembolism (VTE), coupled with increasing preference for novel oral anticoagulants (NOACs) over traditional therapies like warfarin. Key growth factors include strong clinical evidence supporting Eliquis’ superior safety and efficacy, expanding indications, and favorable reimbursement policies in major markets. However, the market faces challenges such as pricing pressures, biosimilar competition in certain regions, and patent expirations post-2026. Emerging opportunities lie in expanding access in developing markets and leveraging real-world data to reinforce value propositions.
- Recent industry events such as the American Heart Association (AHA) Scientific Sessions (2022-2023) and the International Society on Thrombosis and Haemostasis (ISTH) Congress (2023) have highlighted Eliquis’ clinical advancements, reinforcing its market position. Notable initiatives include Bristol-Myers Squibb and Pfizer’s patient assistance programs improving affordability in the U.S., while collaborations with governments in Europe and Asia aim to enhance treatment access. Pilot projects, like Japan’s integrated AFib management programs, are expected to further drive adoption, shaping the future market outlook.
Market Segmentation
- Indication Insights (Revenue, USD Bn, 2020 - 2032)
- Reduction of Risk of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation
- Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
- Deep Vein Thrombosis
- Pulmonary Embolism
- Reduction in the Risk of Recurrence of Deep Vein Thrombosis and Pulmonary Embolism
- Venous Thromboembolism (VTE) and Reduction in the Risk of Recurrent VTE in Pediatric Patients
- Strength Insights (Revenue, USD Bn, 2020 - 2032)
- 5 mg
- 5 mg
- Age Group Insights (Revenue, USD Bn, 2020 - 2032)
- Pediatric
- Adult
- Dosage Form Insights (Revenue, USD Bn, 2020 - 2032)
- Oral Tablet
- Oral Suspension
- Distribution Channel Insights (Revenue, USD Bn, 2020 - 2032)
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Pfizer Inc.
- Bristol-Myers Squibb Company
Sources
Primary Research Interviews
- Interviews with healthcare providers (e.g., rheumatologists, immunologists)
- Interviews with key opinion leaders (KOLs) in the field of autoimmune diseases
- Interviews with pharmacists and medical professionals involved in biologics
- Interviews with patients using Actemra and other biologic therapies
Databases
- National Health Service (NHS) U.K.
- U.S. National Institutes of Health (NIH)
- Centers for Disease Control and Prevention (CDC)
- World Health Organization (WHO)
- European Medicines Agency (EMA)
- National Institute for Health and Care Excellence (NICE)
Magazines
- Pharmaceutical Technology
- BioPharma Reporter
- The Pharmaceutical Journal
Journals
- American Heart Association
- Journal of the American Heart Association (JAHA)
- Journal of Clinical Medicine
- Journal of Stroke and Cerebrovascular Diseases
Newspapers
- The Guardian (UK)
- The New York Times
- The Financial Times
- The Washington Post
- The Times (UK)
- The Wall Street Journal
Associations
- Journal of the American Heart Association (JAHA)
- European Heart Journal (European Society of Cardiology - ESC)
- Heart Rhythm Society
- American Society of Hematology
Public Domain Sources
- U.S. National Library of Medicine
- UK National Health Service (NHS) Resources
- National Health Interview Survey (NHIS)
- Centers for Disease Control and Prevention (CDC) Data
- National Institute for Health and Care Excellence (NICE) Guidelines
Proprietary Elements
- CMI Data Analytics Tool: Proprietary analytics tool to analyze real-time market trends, consumer behavior, and technology adoption in market
- Proprietary CMI Existing Repository of Information for Last 8 Years
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients