GLOBAL CLINICAL LABORATORY TESTS MARKET SIZE AND SHARE ANALYSIS - GROWTH TRENDS AND FORECASTS (2025-2032)
Global Clinical Laboratory Market size is expected to reach USD 369.22 Bn by 2032, from USD 276.83 Bn in 2025, exhibiting a compound annual growth rate (CAGR) of 4.2% during the forecast period.
Key Takeaways
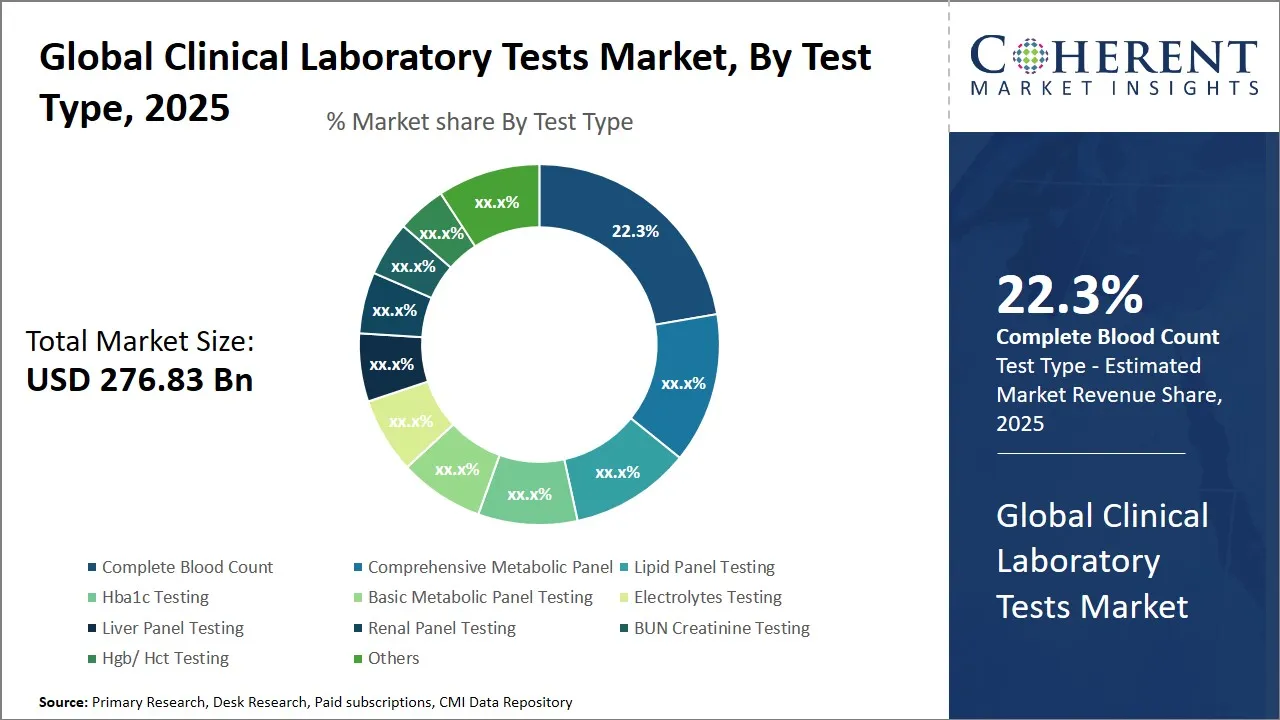
- Based on Test Type, the Complete Blood Count segment is expected to account for 22.3% share in the market in 2025, as it is one of the most fundamental and widely prescribed diagnostic tools.
- Based on Application, the Hematology segment leads the market with the largest share in 2025, owing to the blood-related disorders such as anemia, leukemia, clotting abnormalities, and infections are highly prevalent worldwide.
- Based on End User, the Hospital Laboratories segment is expected to dominated the market with the highest share in 2025, as they handle a large volume of diagnostic tests directly linked to inpatient and outpatient care.
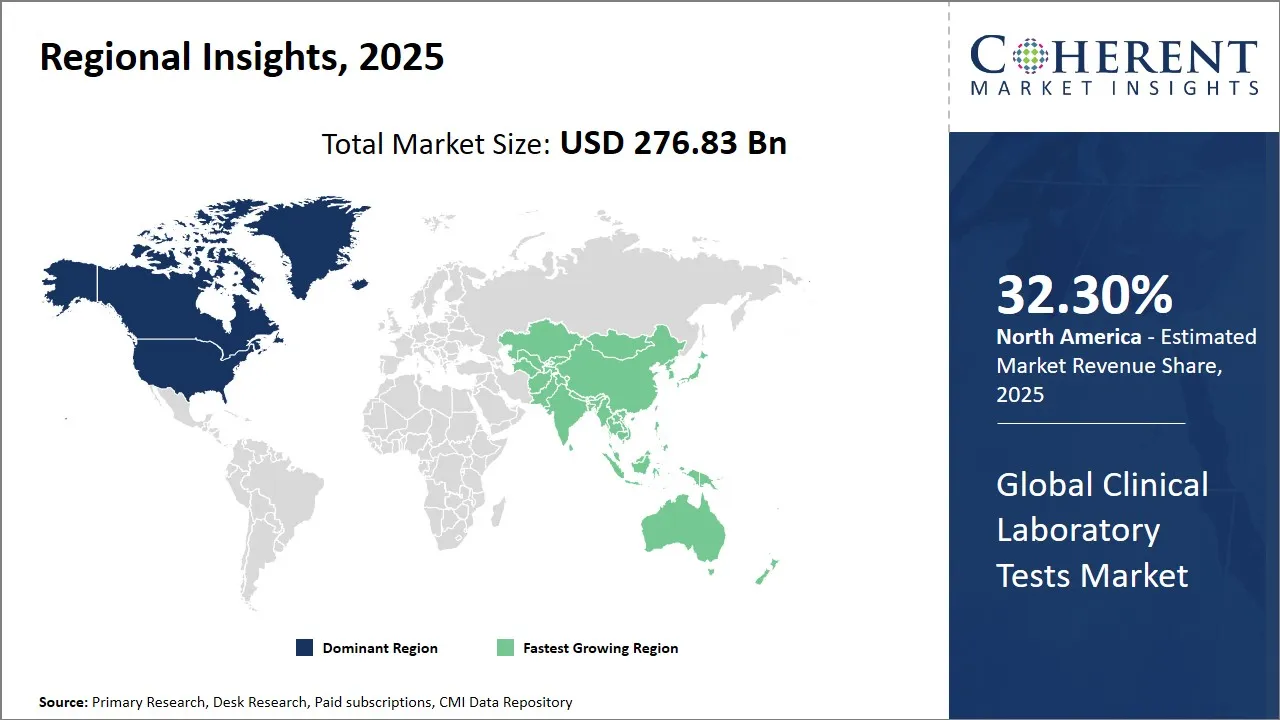
- Based on Region, North America is expected to lead the market, holding a share of 32.30% in 2025. While, Asia Pacific is anticipated to be the fastest-growing region during the forecast period.
Market Overview
The clinical laboratory tests market is witnessing steady growth driven by rising prevalence of chronic and infectious diseases, increasing demand for early diagnosis, and advancements in diagnostic technologies. Growing geriatric population, expanding healthcare infrastructure, and adoption of personalized medicine are further fueling demand, making clinical testing essential for improved patient outcomes.
Current Events and Its Impact
|
Current Event |
Description and its Impact |
|
Regulatory Transformation and Compliance Overhaul |
|
|
Artificial Intelligence and Automation Revolution |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Reimbursement Policy
The reimbursement landscape for the Global Clinical Laboratory Tests Market varies significantly across regions, shaped by national healthcare policies, payer systems, and regulatory frameworks. In the United States, Medicare reimburses most diagnostic laboratory tests through the Clinical Laboratory Fee Schedule (CLFS), with rates updated triennially under the Protecting Access to Medicare Act (PAMA). Private insurers often align with CMS guidelines, negotiating rates individually based on test inclusion in medical policies. In Europe, reimbursement models differ by country: Germany uses fixed rates under the German Diagnosis-Related Groups (G-DRG) system, while the UK’s NHS applies centralized national tariffs adjusted periodically for cost and technological changes. In the Asia-Pacific, countries like Japan and South Korea rely on national health insurance schemes, offering broad coverage, whereas China is gradually integrating innovative diagnostic technologies into its reimbursement framework based on clinical efficacy and cost-effectiveness. Regulatory developments, such as the FDA’s 2023 authorization requirement for Laboratory-Developed Tests (LDTs) in the U.S., further influence reimbursement dynamics.
Role of AI (Artificial Intelligence) In Clinical Laboratory Tests Market
AI is transforming the clinical laboratory tests market by enhancing accuracy, efficiency, and speed of diagnostics. Through machine learning and data analytics, AI can quickly analyze large volumes of lab data, identify patterns, and assist in early disease detection. It enables automation of routine tasks, reduces human error, and supports personalized medicine by interpreting complex biomarkers. AI-powered tools like image recognition in pathology or predictive algorithms in blood tests streamline workflows and improve decision-making.
In May 2025, Niloufer Hospital launched Amruth Swasth Bharath, an AI-based tool developed by Quick Vitals for non-invasive blood testing. Using Remote Photoplethysmography (PPG) and deep learning via camera-enabled devices, it delivers real-time results in under a minute. Designed for the Indian population, it marks a significant step in digitizing public healthcare.
Global Clinical Laboratory Tests Market Insights, By Test Type - Complete Blood Count Leads as it is one of the Most Fundamental and Widely Prescribed Diagnostic Test
In terms of test type, the complete blood count segment, holding an estimated share 22.3% in 2025, dominates the global clinical laboratory tests market, as it is one of the most fundamental and widely prescribed diagnostic tools. CBC helps evaluate overall health and detect a wide range of conditions such as anemia, infections, immune system disorders, and certain cancers. It is frequently used in routine health check-ups, pre-surgical assessments, and ongoing disease monitoring, making it indispensable in both preventive and curative healthcare. Additionally, the rising prevalence of chronic and infectious diseases, along with increasing awareness of early diagnosis, further fuels the global clinical laboratory tests market demand for CBC tests.
For instance, in June 2024, HORIBA introduced three new hematology analyzers, Yumizen H550E (autoloader), H500E CT (closed tube), and H500E OT (open tube) that deliver combined complete blood count (CBC), 5-part white blood cell differential, and erythrocyte sedimentation rate (ESR) results from whole blood in just 60 seconds. Such developments are accelerating the clinical laboratory tests market revenue.
Global Clinical Laboratory Tests Market Insights, By Application - Hematology Dominated Owing to Rising Blood-Related Disorders
In terms of application, the hematology segment is expected to capture the largest share of the market in 2025, owing to the blood-related disorders such as anemia, leukemia, clotting abnormalities, and infections are highly prevalent worldwide. Hematology tests, especially complete blood count (CBC), are routinely prescribed as they provide essential insights into overall health, detect underlying diseases early, and monitor treatment effectiveness. The growing burden of chronic illnesses, rising incidences of cancer, and increasing demand for preventive health check-ups further drive the adoption of hematology testing.
For instance, in December 2024, King Faisal Specialist Hospital & Research Centre (KFSHRC) opened the MENA region’s first Advanced Hematology Diagnostics Laboratory, featuring the fastest automated hematology track, a bulk loader for rapid processing, and AI-powered image analysis. The lab integrates sophisticated pre-analytical workflows and high-resolution multiparametric flow cytometry analyzers, enabling high-precision cellular diagnostics with faster turnaround.
Global Clinical Laboratory Tests Market Insights, By End User - Hospital Laboratories Leads Due to Large Volume of Diagnostics Tests Performed
In terms of end user, the hospital laboratories segment is projected to hold the dominant share in 2025, as they handle a large volume of diagnostic tests directly linked to inpatient and outpatient care. Hospitals rely on in-house labs for timely and accurate results to support critical decision-making in emergency care, surgeries, and chronic disease management. The growing burden of lifestyle-related diseases, rising hospitalization rates, and the integration of advanced diagnostic technologies such as molecular testing and automated analyzers further strengthen hospital laboratories’ dominance.
For instance, in October 2024, City Imaging & Clinical Labs, a leading Indian diagnostic chain currently serving 10 hospitals with 24/7 in-house lab testing and phlebotomy, has announced plans to expand its hospital laboratory management services across North India. Targeting hospitals with 50 or more beds, the company aims to partner with over 50 such facilities by March 2027. The initiative will be supported by advanced LIMS systems, a dedicated corporate quality assurance team, experienced pathologists, and NABL-accredited labs.
Regional Insights
To learn more about this report, Download Free Sample
North America Clinical Laboratory Tests Market Analysis & Trends
North America region is projected to lead the market with a 32.30% share in 2025 due to the region’s advanced healthcare infrastructure, high adoption of preventive diagnostics, and rising prevalence of chronic diseases such as diabetes, cardiovascular disorders, and cancer. Increasing geriatric population, coupled with strong insurance coverage and government support for early disease detection, further drives market demand.
For instance, in April 2025, Nanostics Inc. and Protean BioDiagnostics have announced that the American Medical Association (AMA) has granted a unique Proprietary Laboratory Analyses (PLA) code—0550U to their ClarityDX Prostate test. This code facilitates insurance reimbursement and broadens access to the AI-powered diagnostic tool.
Europe Clinical Laboratory Tests Market Analysis & Trends
Europe market is expected to be the fastest-growing market for clinical laboratory test market, with a share of 19% in 2025. The region has a high prevalence of chronic diseases such as diabetes, cardiovascular disorders, and cancer, driving the need for regular diagnostic testing. Well-established healthcare infrastructure and widespread accessibility to diagnostic laboratories ensure routine and specialized testing is feasible.
For instance, in May 2025, Roche announced a strategic collaboration with Broad Clinical Labs to advance the adoption of its next-generation Sequencing By Expansion (SBX) technology. The partnership aims to explore applications of SBX, focusing initially on trio-based whole genome sequencing for critically ill newborns and their biological parents. This initiative seeks to integrate whole genome sequencing into routine clinical care in neonatal intensive care units (NICUs), enabling timely and precise diagnoses for infants with suspected genetic disorders.
Clinical Laboratory Tests Market Outlook Country-Wise
The U.S. Clinical Laboratory Tests Market Trends
The global clinical laboratory tests market is highly demanding in the U.S. due to the country’s advanced healthcare infrastructure, high prevalence of chronic and lifestyle-related diseases, and strong emphasis on preventive healthcare. Widespread adoption of routine diagnostic testing, early disease detection, and personalized medicine drives consistent demand. Additionally, growing awareness among patients, expanding clinical research, and technological advancements in automated and high-throughput laboratory testing further fuel market growth in the U.S., making it one of the largest and most mature markets globally.
For instance, in May 2025, The U.S. Food and Drug Administration (FDA) has approved the blood test to aid in diagnosing Alzheimer's disease. The Lumipulse G pTau217/β-Amyloid 1-42 Plasma Ratio measures two proteins—pTau217 and β-amyloid 1-42—in blood plasma to detect amyloid plaques in the brain, a hallmark of Alzheimer's. This test offers a less invasive alternative to traditional positron emission tomography (PET) scans, which are costly and expose patients to radiation.
Germany Clinical Laboratory Tests Market Trends
The clinical laboratory tests market in Germany is witnessing strong demand due to multiple factors. Rising incidence of chronic diseases such as diabetes, cardiovascular conditions, and cancer is driving the need for regular diagnostic testing to support disease management. An aging population further increases demand, as older individuals require more frequent monitoring. Adoption of advanced technologies, including automation and artificial intelligence, is improving the efficiency and accuracy of tests, enhancing patient outcomes.
For instance, in April 2025, Biogen and Eisai have launched LEQEMBI® (lecanemab) in Austria and Germany, marking the first European Union markets for the anti-amyloid beta monoclonal antibody. LEQEMBI is the first therapy targeting an underlying cause of Alzheimer's disease. It is indicated for adults with mild cognitive impairment or mild dementia due to Alzheimer's, who are non-carriers or heterozygotes of the ApoE ε4 gene with confirmed amyloid pathology.
Market Report Scope
Clinical Laboratory Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 276.83 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.2% | 2032 Value Projection: | USD 369.22 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
ARUP Laboratories, OPKO Health, Inc., UNILABS, Clinical Reference Laboratory, Inc., Synnovis Group, LLP, Sonic Healthcare Limited., Quest Diagnostics Incorporated.,Abbott, Cinven, Laboratory Corporation of America Holdings, Neogenomic Laboratories, Inc., Fresenius Medical Care, Eurofins Scientific., Qiagen N. V., and Life Lab |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Dynamics
Global Clinical Laboratory Tests Market Drivers
- Rising burden of chronic diseases: With increasingly sedentary lifestyles and unhealthy eating habits becoming prevalent across the world, chronic diseases have been on a rise over the past few decades. Chronic conditions like cancer, cardiovascular diseases, diabetes, and others have emerged as the leading causes of mortality and reduced quality of life in majority of the countries. These diseases require long term medical supervision and a series of laboratory tests to monitor the condition of patients and assess treatment effectiveness over time. Rising cases of chronic diseases directly translate to increased demand for clinical pathology tests from hospitals and diagnostic laboratories. More tests mean more business and revenues for labs. Even developing nations are experiencing growing rates of non-communicable diseases as rates of urbanization increase and traditional diets change. This growing chronic disease burden faced by healthcare systems globally is a key driver positively impacting the clinical laboratory tests market.
Global Clinical Laboratory Tests Market Trends
- Technological Advancement such as In-vitro Diagnostics: Technological advancements in the field of in-vitro diagnostics have revolutionized clinical pathology tests in recent years. Introduction of automated, high-throughput solutions have made testing faster, more accurate and affordable. Automated analyzers, microfluidics based solutions, point-of-care (POC) testing devices and molecular diagnostic platforms are helping clinicians to obtain diagnostic information quickly and facilitate prompt treatment decisions. Adoption of advanced testing methods like immunohistochemistry, flow cytometry, genetic testing, and others have expanded testing options well beyond traditional clinical chemistry and immunoassays.
Analyst Opinion (Expert Opinion)
The global clinical laboratory tests market is experiencing a transformative phase characterized by rapid technological advancements, evolving regulatory landscapes, and shifting healthcare paradigms. These factors collectively influence the market's trajectory and underscore the need for strategic adaptation by stakeholders.
The integration of automation and artificial intelligence (AI) into laboratory operations is revolutionizing test accuracy, throughput, and cost-efficiency. For instance, the adoption of AI-driven diagnostic platforms enables real-time data analysis, facilitating early disease detection and personalized treatment plans. This technological evolution is not merely a trend but a fundamental shift that enhances the value proposition of clinical laboratory services.
Regulatory frameworks play a pivotal role in shaping the clinical laboratory tests market. Recent developments indicate a potential rollback of stringent FDA regulations on laboratory-developed tests (LDTs) in the United States. Such policy changes could lead to increased market entry of new diagnostic tests, potentially affecting market dynamics and pricing structures. Stakeholders must remain vigilant and adaptable to navigate these regulatory shifts effectively.
Global Clinical Laboratory Tests Market- Recent Developments
- In May 2025, Apollo Diagnostics inaugurated its fully automated Digi-Smart Central Reference Laboratory in Chennai, spanning 45,000 square feet. The state-of-the-art facility integrates five major lab disciplines—Clinical Chemistry, Immunoassay, Serology, Haematology, and Haemostasis, into a unified, digitally monitored system. Employing robotics, high-definition cameras, machine learning technologies, and proprietary algorithms, the lab aims to reduce sample turnaround time by 60%, processing over 100,000 samples daily.
- In May 2025, ai introduced a groundbreaking machine learning platform designed to enhance real-time PCR diagnostics. This platform, known as PCR.AI, offers unprecedented transparency by clearly demonstrating how each diagnostic result is achieved, addressing the longstanding issue of AI "black boxes" in medical diagnostics.
Market Segmentation
- Global Clinical Laboratory Tests Market, By Test Type
- Complete Blood Count
- Hgb/ Hct Testing
- Basic Metabolic Panel Testing
- BUN Creatinine Testing
- Electrolytes Testing
- Hba1c Testing
- Comprehensive Metabolic Panel
- Liver Panel Testing
- Renal Panel Testing
- Lipid Panel Testing
- Others
- Global Clinical Laboratory Tests Market, By Application
- Parasitology
- Virology
- Hematology
- Toxicology
- Immunology/serology
- Histopathology And Urinalysis
- Global Clinical Laboratory Tests Market, By End User
- Central Laboratories
- Hospital Laboratories
- Independent Laboratories
- Research and Academia
- Global Clinical Laboratory Tests Market, By Region
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Top Companies in the Global Clinical Laboratory Tests Market
- ARUP Laboratories
- OPKO Health, Inc.
- UNILABS
- Clinical Reference Laboratory, Inc.
- Synnovis Group, LLP
- Sonic Healthcare Limited.
- Quest Diagnostics Incorporated.
- Abbott
- Cinven
- Laboratory Corporation of America Holdings
- Neogenomic Laboratories, Inc.
- Fresenius Medical Care
- Eurofins Scientific
- Qiagen N. V.
- Life Lab
Sources
Primary Research Interviews from the following stakeholders
Stakeholders
- Interviews with hospital laboratory heads, diagnostic center managers, clinical chemists, pathologists, molecular biologists, and procurement heads across leading global markets.
Specific stakeholders
- Laboratory directors and heads of diagnostics at multi-specialty hospitals (e.g., Apollo Hospitals, Mayo Clinic, Cleveland Clinic)
- Operations and quality control managers at central reference laboratories
- Procurement and supply chain managers at diagnostic service providers
- IT and laboratory automation heads in hospital networks
- Product managers at clinical diagnostic equipment manufacturers (e.g., analyzers, reagents, lab automation systems)
- Research heads in biotechnology and molecular diagnostics labs
Databases
- World Health Organization (WHO) Health Statistics
- UN Comtrade Database
- Centers for Disease Control and Prevention (CDC), U.S.
- European Centre for Disease Prevention and Control (ECDC)
- National Health Service (NHS) Digital Data, UK
- Ministry of Health and Family Welfare (MoHFW), India
- China National Health Commission Data Portal
- Korea Disease Control and Prevention Agency (KDCA)
Magazines
- Clinical Laboratory News
- Lab Manager Magazine
- The Scientist – Laboratory Technology Section
- Medical Laboratory Observer (MLO)
- Laboratory Equipment Magazine
- BioTechniques
Journals
- Clinical Chemistry
- Journal of Clinical Laboratory Analysis
- Annals of Clinical Biochemistry
- Laboratory Medicine
- Journal of Laboratory Automation
- Clinical Biochemistry
Newspapers
- The Wall Street Journal – Healthcare & Diagnostics
- The Economic Times – Healthcare & Biotechnology
- The Hindu Business Line – Health Tech & Labs
- Financial Times – Healthcare & Diagnostics
- Nikkei Asia – Medical Technology & Diagnostics
Associations
- American Association for Clinical Chemistry (AACC)
- International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)
- Clinical Laboratory Management Association (CLMA)
- Association for Molecular Pathology (AMP)
- Indian Association of Clinical Biochemists (IACB)
- European Federation of Clinical Chemistry and Laboratory Medicine (EFLM)
Public Domain Sources
- U.S. Food & Drug Administration (FDA) – Diagnostics & Laboratory Guidelines
- National Institutes of Health (NIH)
- Ministry of Health and Family Welfare (MoHFW), India
- National Institute of Standards and Technology (NIST), U.S.
- Centers for Medicare & Medicaid Services (CMS) – Laboratory Data
- World Health Organization (WHO) – Diagnostics and Laboratory Reports
Proprietary Elements
- CMI Data Analytics Tool, and Proprietary CMI Existing Repository of information for last 8 years
*Definition: Clinical laboratory tests play a pivotal role in disease diagnosis and health management. Various biochemical, hematological, immunological, histopathological and microbiological tests are conducted in clinical laboratories to detect infections, screen for chronic conditions, monitor disease progression and check treatment effectiveness.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients