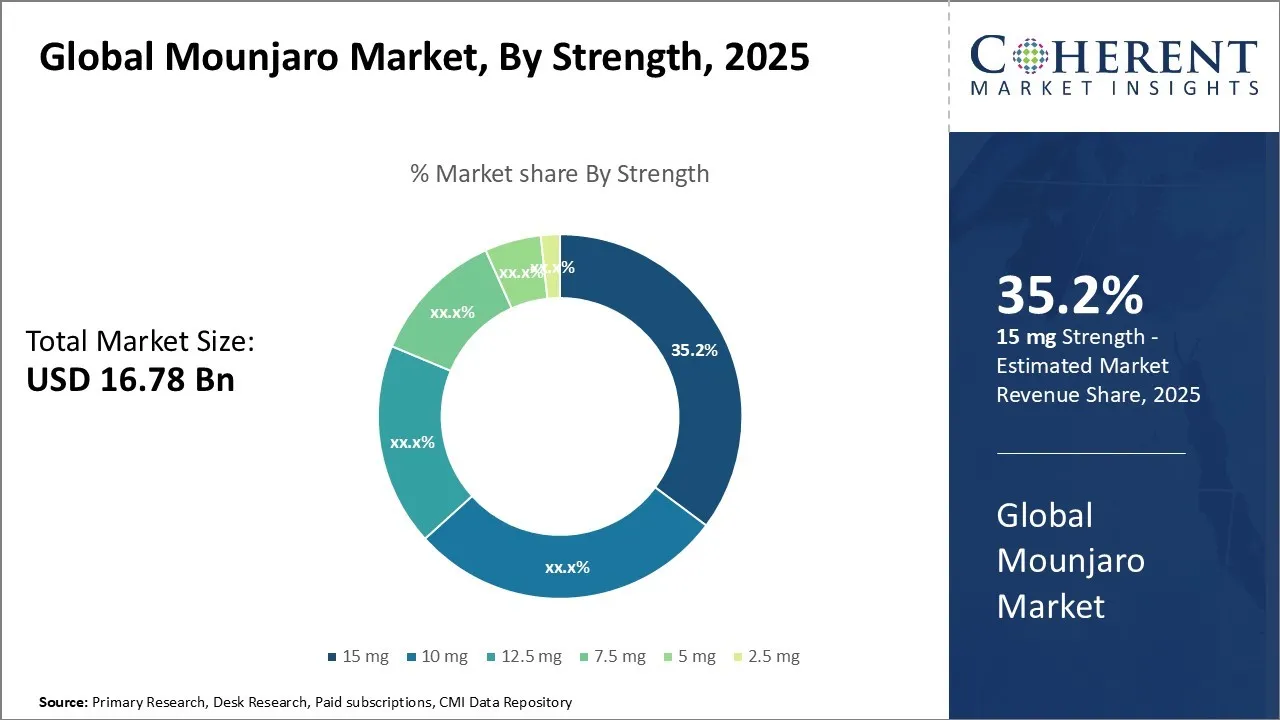
Global Mounjaro Market Size and Forecast
The Global Mounjaro Market is estimated to be valued at USD 16.78 Bn in 2025 and is expected to reach USD 55.48 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 18.6% from 2025 to 2032. This significant growth reflects increasing adoption and demand across various regions, underscoring strong market potential over the forecast period.
Key Takeaways of the Global Mounjaro Market
- In the strength segment, the 15 mg dosage holds the largest market share, expected to account for 35. 2% in 2025.
- As per dosage form, the single-dose pen segment is expected to dominate, contributing 83. 1% to the global Mounjaro market in 2025.
- North America is expected to lead the market, holding a share of 37. 3% in 2025. Asia Pacific is anticipated to be the fastest-growing region, with a market share of 25.5% in 2025.
Market Overview
Mounjaro (tirzepatide), developed by Eli Lilly, has emerged as a leading treatment for type 2 diabetes and obesity, leveraging its unique dual GLP-1 and GIP agonist mechanism. Approved by the U.S. FDA in 2022 for diabetes and later for chronic weight management, Mounjaro has gained rapid adoption due to its superior efficacy in reducing blood sugar and promoting significant weight loss compared to older GLP-1 drugs like semaglutide (Ozempic/Wegovy). The drug is primarily administered via single-dose auto-injector pens, which dominate the market due to their ease of use and patient preference. Prescriptions are heavily concentrated in higher doses (10 mg, 12.5 mg, and 15 mg), as these strengths deliver optimal therapeutic effects for long-term weight management and glycemic control. While adult patients account for the vast majority of usage, pediatric adoption remains minimal due to limited approvals. Mounjaro faces strong competition from Novo Nordisk’s semaglutide-based therapies but maintains an edge in clinical outcomes, fueling its widespread use in both diabetes and obesity care.
Currents Events and their Impact
|
Current Events |
Description and its impact |
|
Approval of Mounjaro in Emerging Countries |
|
|
Revenue Increased of Mounjaro in first quarter, 2025 |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Mounjaro Market Insights, By Strength – 15 mg contributes the highest share of the market owing to its optimal therapeutic efficacy and patient adherence benefits
In the global Mounjaro market, the 15 mg strength emerges as the leading segment with an estimated 35.2% market share in the year 2025. This dominance can be directly attributed to its well-recognized balance between efficacy and safety, making it a preferred choice among healthcare providers and patients alike. The 15 mg dosage is often considered the optimal therapeutic dose as it delivers maximum clinical benefits while minimizing the risk of adverse effects, which is critical inpatient management and long-term treatment adherence. Patients requiring higher therapeutic potency often find the 15 mg dosage especially advantageous. It is known to provide superior glycemic control and weight reduction benefits, addressing key treatment targets efficiently. In clinical settings, the higher strength enables physicians to tailor treatment plans that are more aggressive yet manageable, particularly for patients who have not achieved desired results on lower doses. This flexibility spurs confidence in prescribers to initiate or escalate treatment while preserving patient safety.
The convenience factor associated with a higher dose that can reduce the frequency or complexity of adjustments also plays a role in the 15 mg strength preference. Higher doses minimize treatment regimen changes, simplifying patient experience and improving compliance, which is crucial in chronic diseases management like type 2 diabetes or obesity.
Mounjaro Market Insights, By Dosage Form - Single-dose Pen dominates by enhancing patient convenience and improving adherence in outpatient settings
Among the dosage form segment within the global Mounjaro market, the single-dose pen segment is notably the market leader with an estimated 83.1% market share for the year 2025, driven largely by patient-centric factors that prioritize ease of use, dosage accuracy, and mobility. The single-dose pen format addresses several significant challenges faced by patients and healthcare providers, particularly in outpatient and home-use scenarios. The foremost advantage of the single-dose pen is its user-friendly design, which simplifies the administration process. Unlike traditional vials, the pen devices are pre-filled, eliminating the need for measuring and drawing medication, thereby reducing errors and improving dosing precision. This design is particularly helpful for patients with limited dexterity, visual impairments, or those new to injectable treatments. Such convenience encourages consistent adherence, as patients are less likely to skip doses due to complexity or fear of incorrect administration.
Portability and discreetness of the single-dose pen further enhance its appeal. Patients leading active lifestyles or requiring frequent mobility find the pens easier to carry and use discreetly in social or work settings without drawing attention. This level of flexibility improves patient confidence in managing their condition effectively outside clinical environments, resulting in better health outcomes over time. Healthcare systems and providers also appreciate the single-dose pen’s capacity to reduce waste and simplify inventory management, contributing indirectly to lowering treatment costs and fostering sustainable healthcare practices. These collective advantages create a compelling case for the single-dose pen’s dominance as the dosage form of choice, amplifying its share within the global Mounjaro market.
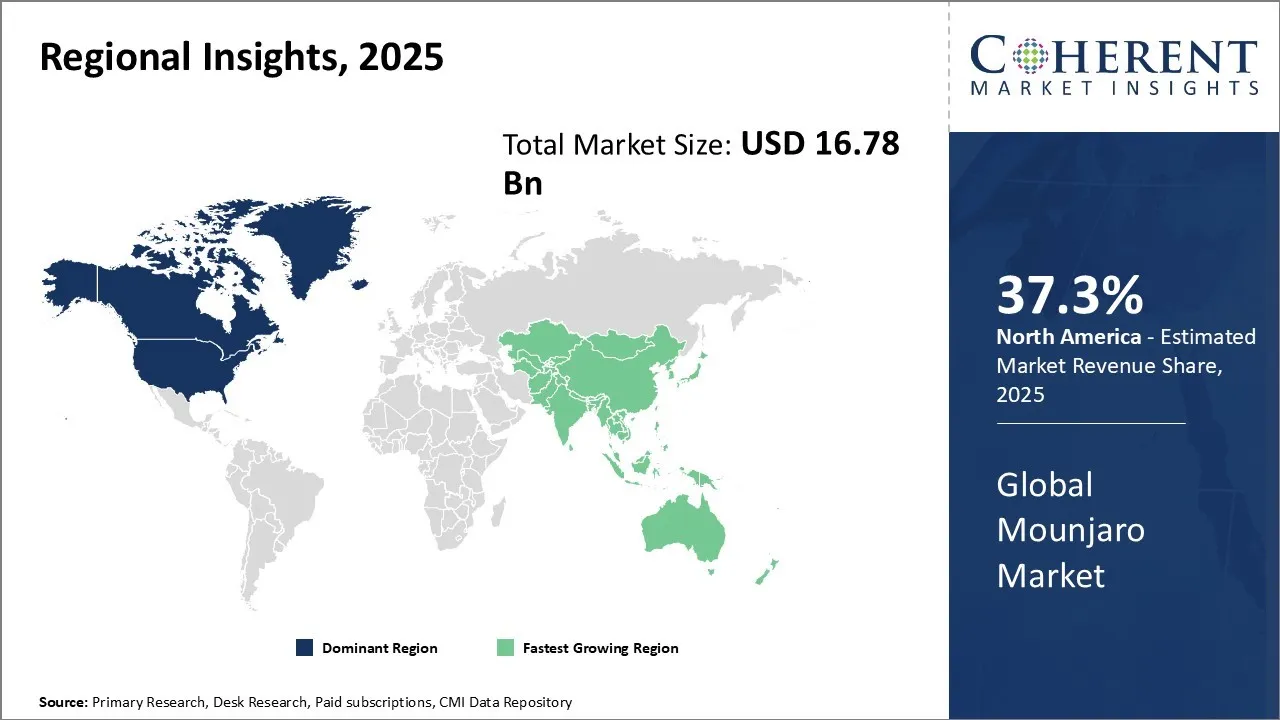
Regional Insights
To learn more about this report, Download Free Sample
North America Mounjaro Market Analysis and Trends
The North America is expected to hold 37.3% market share in the global Mounjaro market. The dominance in the global Mounjaro market is driven by a mature healthcare infrastructure, high awareness of advanced treatment options, and strong industry presence. The region benefits from robust government policies supportive of pharmaceutical innovation, including streamlined regulatory frameworks and incentivization of research and development. Moreover, the launch of Mounjaro and approval has significantly impacted the market. For instance, in May 2022, the U.S. Food and Drug Administration (FDA) approved Mounjaro (tirzepatide) injection, Eli Lilly and Company's new once-weekly GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
Asia Pacific Mounjaro Market Analysis and Trends
The Asia Pacific region exhibits the fastest growth with market share of 25.5% in 2025 in the global Mounjaro market, propelled by increasing healthcare expenditure, rising prevalence of chronic diseases, and expanding patient awareness in emerging economies. Governments across the region are enhancing healthcare infrastructure and introducing regulatory reforms to facilitate faster drug approvals. Furthermore, increasing adoption of growth strategies such as launch of Mounjaro in Asian countries is expected to drive the market in the Rregion. For instance, on January 2, 2025, Eli Lilly announced that its self-developed GIP/GLP-1 receptor dual agonist tirzepatide injection, Mounjaro, was officially launched in China. Mounjaro covers two indications "type 2 diabetes" and "weight loss," which were approved by the National Medical Products Administration in May and July 2024, respectively.
Global Mounjaro Market Outlook for Key Countries
U.S. Mounjaro Market Analysis and Trends
The U.S. Mounjaro market remains at the forefront due to its high healthcare spending and early adoption of innovative therapies like Mounjaro. Major pharmaceutical companies headquartered here, including Eli Lilly and Novo Nordisk, actively drive clinical research and extensive marketing campaigns. The U.S. healthcare ecosystem, characterized by strong payer systems and advanced hospital networks, supports widespread usage. Government programs focusing on diabetes and obesity management further amplify demand, making the country a pivotal market in the global landscape.
Germany Mounjaro Market Analysis and Trends
Germany’s Mounjaro market benefits from its strong healthcare system and stringent regulatory environment that ensures high safety and efficacy standards. The country hosts significant operations of multinational pharma firms that facilitate efficient distribution and localized clinical research for Mounjaro. Germany’s reimbursement framework supports innovative treatments, encouraging physician uptake. Furthermore, Mounjaro was approved in Germany. After the approval Eli Lilly and the company announced a massive USD 2.7 Billion (EUR 2.3 billion) expansion of its German facilities in 2023.
Japan Mounjaro Market Analysis and Trends
Japan continues to lead in adoption due to its obese population with a high incidence of metabolic disorders, coupled with progressive healthcare policies. The government’s focus on combating lifestyle diseases aligns with increased use of medications like Mounjaro. Japan’s pharmaceutical industry is marked by collaboration between domestic companies and international entities, fostering effective market penetration. Efficient regulatory pathways and strong intellectual property protections also support ongoing product innovation and availability. Moreover, in September 2022, the Ministry of Health, Labour and Welfare (MHLW) approved the manufacturing and marketing of Mounjaro, a sustained-release GIP/GLP-1 receptor agonist, for the treatment of type 2 diabetes in Japan. The approved formulation is a subcutaneous injection available in six dosages 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg ATEOS, with the non-proprietary name Tirzepatide (referred to as "Mounjaro"). This approval introduces Mounjaro as a new therapeutic option, expanding treatment alternatives for patients with type 2 diabetes in the Japanese market.
China Mounjaro Market Analysis and Trends
China’s Mounjaro market is rapidly expanding, supported by its sizeable patient population and vigorous government initiatives aimed at improving diabetes care. Regulatory reforms have accelerated drug approvals, and strategic partnerships between foreign pharmaceutical companies and local manufacturers enhance distribution channels. The growing emphasis on health awareness and increased insurance coverage have driven patient accessibility. Investments in digital health platforms further facilitate patient engagement and adherence, positioning China as a critical growth market.
India Mounjaro Market Analysis
The India Mounjaro market is estimated to be valued at USD 2.8 Mn in 2025 and is expected to reach USD 7.4 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 15.2% from 2025 to 2032.
India Market Trends
- The Indian market has seen a surge in demand for Mounjaro (tirzepatide), a dual GIP and GLP-1 receptor agonist, primarily driven by rising obesity and type 2 diabetes cases. With increasing awareness about its weight loss benefits, pharmacies and online platforms are reporting higher sales, especially in metro cities like Mumbai, New Delhi, and Bangalore. The drug, though priced at a premium, is gaining traction among affluent patients and those seeking advanced diabetes management solutions.
- Despite growing demand, Mounjaro faces intermittent supply shortages in India due to import dependencies and regulatory hurdles. Stockouts in major hospitals and retail pharmacies have been reported, leading to price fluctuations in the gray market. Eli Lilly, the manufacturer, is working to stabilize supplies, but patients are currently facing delays and higher costs, with some opting for alternative GLP-1 agonists like semaglutide (Ozempic) as temporary substitutes.
India Specific Drug Approved Matrix
|
Category |
Details |
|
Generic Name |
Tirzepatide |
|
Brand Names (India) |
Mounjaro |
|
Therapeutic Class |
GIP/GLP-1 receptor agonist |
|
Approved Indications |
Type 2 Diabetes (Off label Obesity) |
|
Dosage Forms (Approved) |
2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg and 15 mg |
|
DCGI Approval Date |
March 2025 |
|
Key Manufacturers |
Eli Lilly |
|
MRP Range (₹) |
4,375 rupees (USD50.67) for a 5 mg vial and 3,500 rupees (USD40.54) for a 2.5 mg vial |
|
OTC/Rx Status |
Prescription-only (Rx) |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Mounjaro Cost in India Current Pricing and Future Outlook
- Accessibility is a key factor. Currently, Mounjaro’s pricing in India is reported as
- Starter Dose (2.5mg) Approximately ₹3,500 per vial (weekly injection).
- Maintenance Dose (e.g., 5mg) Approximately ₹4,375 per vial.
- This translates to a monthly cost ranging from roughly ₹14,000 (for the lowest dose) to potentially ₹17,500 or more as doses increase. The estimated annual cost could easily exceed ₹2 lakh, placing it out of reach for a large segment of the population.
- However, there’s a potential shift on the horizon. The patent for tirzepatide is expected to expire around 2026. Following patent expiry, generic versions could enter the market, potentially drastically reducing the cost – perhaps to around ₹4,000 per month, making it significantly more accessible.
Physician Perspective on Mounjaro (Tirzepatide) in India
- Mounjaro (tirzepatide) has garnered significant attention in India’s healthcare landscape due to its potential to induce substantial weight loss—up to 20%, without requiring extreme dietary restrictions or rigorous exercise regimens. Given India’s escalating burden of obesity and type 2 diabetes, this drug presents a promising therapeutic option. However, as physicians, it is essential to maintain a cautious and evidence-based approach when evaluating its role in patient care.
- While the clinical benefits of Mounjaro, including improved glycemic control and significant weight reduction, are well-documented, its use must be carefully weighed against potential risks. The drug’s side effect profile, particularly gastrointestinal disturbances such as nausea, vomiting, and diarrhea, can be severe for some patients. Additionally, concerns about muscle loss in the absence of structured nutritional and physical activity support warrant close monitoring.
- Another critical consideration is affordability. At present, Mounjaro remains a high-cost therapy, limiting access for a large segment of the Indian population. This raises ethical questions about equitable treatment options, especially when lifestyle modifications and other pharmacological alternatives may offer sustainable benefits at lower costs.
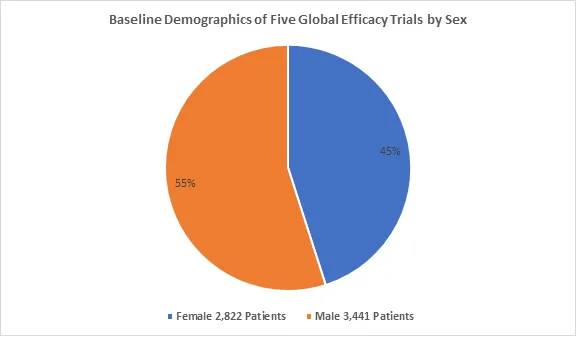
Drug Trials Snapshot Summary of Mounjaro
- The U.S. FDA approved MOUNJARO based on evidence from nine clinical trials of 7,769 patients with type 2 diabetes mellitus, of which 5,415 of these patients received MOUNJARO. The trials were conducted at 673 sites in 24 countries, including Argentina, Australia, Brazil, Canada, India, Israel, Japan, Mexico, Russian Federation, South Korea, Taiwan, multiple European countries, and the U.S. (including Puerto Rico). All nine trials were used to assess safety and five of these trials were used to assess the efficacy of Mounjaro. The five trials used in the efficacy evaluation included 6,263 adult patients with type 2 diabetes mellitus. Four additional trials were included in the safety evaluation, for a total of 7,769 adult patients with type 2 diabetes; therefore, the number of patients representing efficacy findings may differ from the number of patients representing safety findings due to different pools of study participants analyzed for efficacy and safety.
- Below summarizes how many female and male patients were enrolled in the five combined clinical trials used to evaluate the efficacy of Mounjaro.
To learn more about this report, Download Free Sample
Market Players, Key Developments, and Competitive Intelligence

To learn more about this report, Download Free Sample
Key Developments
- On June 26, 2025, Eli Lilly and Company announced the launch of pre-filled injector pens of its blockbuster weight-loss drug, Mounjaro. Lilly started selling Mounjaro in India in late March for diabetes and obesity, and it was so far available only in 2.5 mg and 5 mg vials. Mounjaro KwikPen, for once-weekly use, has been approved by the Central Drugs Standard Control Organization for six dose strengths of 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg and 15 mg. Lilly Canada announced that Mounjaro (tirzepatide) is available in Canada in November 2023. Mounjaro is a new once-weekly GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptor agonist, and is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
- In May 2022, the U.S. Food and Drug Administration (FDA) approved Mounjaro (tirzepatide) injection, Eli Lilly and Company's new once-weekly GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes. Mounjaro has not been studied in patients with a history of pancreatitis and is not indicated for use in patients with type 1 diabetes mellitus.
Market Report Scope
Mounjaro Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 16.78 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 18.6% | 2032 Value Projection: | USD 55.48 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Eli Lilly and Company |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
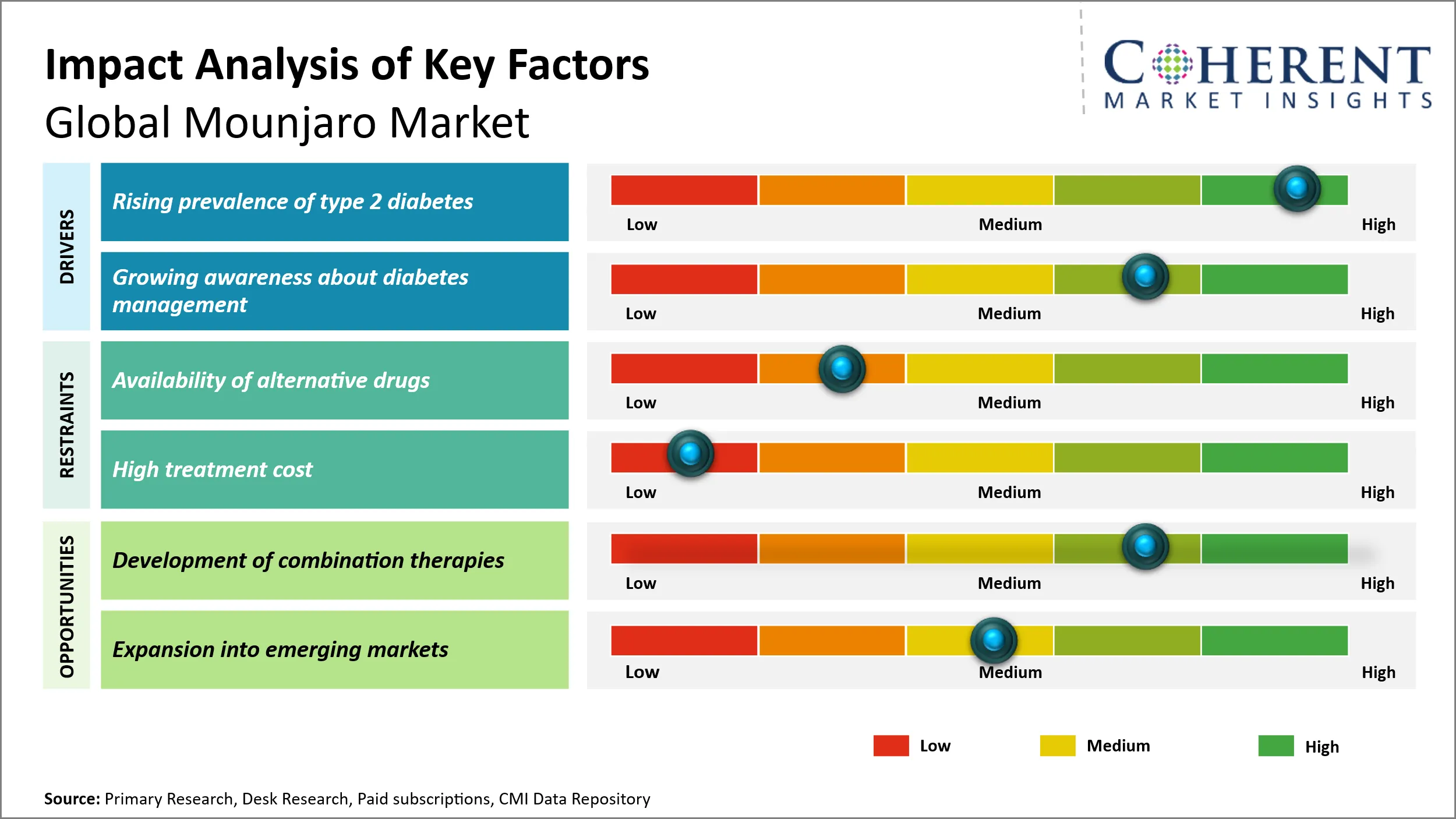
Mounjaro Market Dynamics
To learn more about this report, Download Free Sample
Market Driver - Rising Prevalence of Type 2 Diabetes
The escalating prevalence of type 2 diabetes worldwide is a critical factor driving the demand for Mounjaro, a medication designed to manage blood sugar levels effectively in diabetic patients. For instance, International Diabetes Federation in 2024 provided a data according to which by 2050, projections show that 1 in 8 adults, approximately 853 million, will be living with diabetes, an increase of 46% and over 90% of people with diabetes have type 2 diabetes. The prevalence is driven by socio-economic, demographic, environmental, and genetic factors. Increasing urbanization, sedentary lifestyles, and poor dietary habits are contributing significantly to the surge in type 2 diabetes cases, especially in developing and developed regions alike.
As the population ages and risk factors such as obesity continue to rise, more individuals are being diagnosed with this chronic condition, necessitating improved therapeutic options. Mounjaro’s effectiveness in controlling glycemic levels and reducing associated complications makes it a preferred choice among healthcare providers and patients. Additionally, the growing awareness about managing and preventing diabetes-related complications further accentuates the importance of treatments like Mounjaro. This expanding patient base with unmet medical needs amplifies the demand for innovative and efficient diabetes treatments, positioning Mounjaro favorably in a market shaped by a persistent increase in type 2 diabetes prevalence.
Market Opportunity - Expansion into Emerging Markets
the global Mounjaro market is poised for growth through strategic expansion into emerging markets. A key development was its approval and launch in India in September 2023, marking a significant entry into a high-potential market with a rising diabetes and obesity burden. Similarly, Brazil’s ANVISA approved Mounjaro in Q1 2024, with Eli Lilly partnering with local distributors to accelerate patient access. These launches demonstrate the drug’s increasing global footprint, particularly in regions where metabolic disorders are surging. Further opportunities exist in Southeast Asia and the Middle East, where regulatory filings are underway. By leveraging these expansions, Eli Lilly can tap into underserved populations, supported by evolving healthcare policies and growing demand for innovative therapies.
Analyst Opinion (Expert Opinion)
- The global Mounjaro market has witnessed exponential growth, driven by its dual approval for type 2 diabetes and obesity in key markets like the U.S. and Europe. According to the International Diabetes Federation (IDF), India has over 101 million diabetic patients, with obesity rates climbing rapidly, making it a high-potential market for GLP-1/GIP agonists. However, Eli Lilly’s pricing strategy and import dependency have restricted Mounjaro’s uptake in India, where out-of-pocket healthcare expenditure remains a critical barrier. Analysts project that local manufacturing or price adjustments could accelerate adoption, but until then, semaglutide and domestic alternatives will dominate the Indian market.
- The drug’s superior efficacy, demonstrating up to 22.5% weight loss in clinical trials, has solidified its dominance in premium markets, particularly among patients seeking rapid and sustained metabolic benefits. However, supply constraints and high production costs remain challenges, with Bloomberg Intelligence noting that Eli Lilly is investing heavily in expanding manufacturing capacity to meet surging global demand. Regulatory approvals in emerging markets like China and Brazil are expected to further fuel growth, though pricing strategies will be critical in these cost-sensitive regions.
Market Segmentation
- Strength Insights (Revenue, USD Bn, 2022 - 2032)
- 15 mg
- 10 mg
- 5 mg
- 5 mg
- 5 mg
- 5 mg
- Dosage Form Insights (Revenue, USD Bn, 2022 - 2032)
- Single-dose Pen
- Single-dose Vial
- Distribution Channel Insights (Revenue, USD Bn, 2022 - 2032)
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Regional Insights (Revenue, USD Bn, 2022 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Eli Lilly and Company
Sources
Primary Research Interviews
- Interviews with healthcare providers (e.g., Diabetologist, immunologists)
- Interviews with key opinion leaders (KOLs)
- Interviews with pharmacists and medical professionals
- Interviews with patients using Mounjaro
Databases
- National Health Service (NHS) U.K.
- U.S. National Institutes of Health (NIH)
- Centers for Disease Control and Prevention (CDC)
- World Health Organization (WHO)
- European Medicines Agency (EMA)
- National Institute for Health and Care Excellence (NICE)
Magazines
- Pharmaceutical Technology
- BioPharma Reporter
- The Pharmaceutical Journal
Journals
- American Diabetes Association (ADA)
- BMJ Open Diabetes Research & Care
- Journal of Diabetes and Metabolic Disorders
- JMIR Diabetes
Newspapers
- The Guardian (UK)
- The New York Times
- The Financial Times
- The Washington Post
- The Times (UK)
- The Wall Street Journal
Associations
- American Diabetes Association
- International Diabetes Federation (IDF)
- Diabetes Care & Education
- World Diabetes Foundation
- American Association of Clinical Endocrinologists
Public Domain Sources
- U.S. National Library of Medicine
- UK National Health Service (NHS) Resources
- National Health Interview Survey (NHIS)
- Centers for Disease Control and Prevention (CDC) Data
- National Institute for Health and Care Excellence (NICE) Guidelines
Proprietary Elements
- CMI Data Analytics Tool Proprietary analytics tool to analyze real-time market trends, consumer behavior, and technology adoption in market
- Proprietary CMI Existing Repository of Information for Last 8 Years
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients