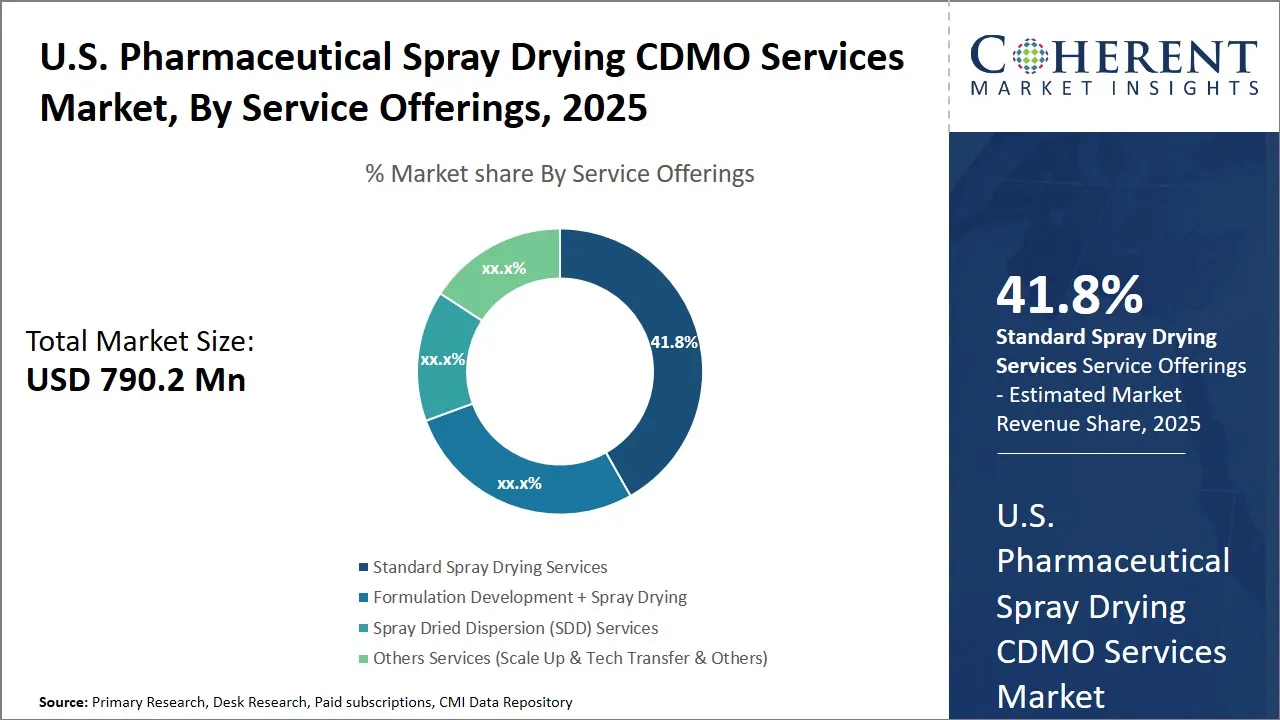
U.S. Pharmaceutical Spray Drying CDMO Services Market Size and Forecast – 2025 to 2032
The U.S. Pharmaceutical Spray Drying CDMO Services Market is estimated to be valued at USD 790.2 Mn in 2025 and is expected to reach USD 1,345.5 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 7.9% from 2025 to 2032. This significant growth reflects increasing demand for advanced drug formulation technologies, driven by the pharmaceutical industry's focus on improving drug bioavailability and stability through innovative spray drying solutions.
Key Takeaways of the U.S. Pharmaceutical Spray Drying CDMO Services Market
- Standard spray drying services segment is expected to account for the largest share of the U.S. pharmaceutical spray drying CDMO services market, representing 41.8%share in 2025.
- Small molecules and APIs segment is projected to lead the market by product, capturing 42.1% share in 2025.
- Pilot/clinical-scale services remain the dominant operational scale segment, commanding an estimated 78.5% of the market share in 2025.
Market Overview
A key market trend is the rising adoption of spray drying technology for producing inhalable drug formulations and particle engineering, which enhances drug delivery efficiency. Additionally, advancements in continuous spray drying processes and the expansion of biopharmaceutical applications are driving the market’s evolution. Increasing investments in research and development, alongside the growing prevalence of chronic diseases, further propel the demand, positioning spray drying as a critical process in modern pharmaceutical manufacturing.
Current Events and Its Impact
|
Current Events |
Description and its Impact |
|
Advancement and Commercialization of mRNA & Gene Therapies |
|
|
Reshoring push and new incentives for domestic drug manufacturing |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
U.S. Pharmaceutical Spray Drying CDMO Services Market Insights, By Service Offerings – Dominance of Standard Spray Drying Services is Driven by Cost Efficiency and Versatility
The standard spray drying services are likely to have the highest market share of the U.S pharmaceutical spray drying CDMO services market at 41.8% share in 2025, due to their reliability, effective operation, and their suitability in diverse pharmaceutical formulations.
Spray drying is an essential process of converting liquid drug preparations into solid, dry, stable powders with increased bioavailability and physical manipulability. Indicatively, business organizations like Cambrex, Quotient Sciences, and Lubrizol Life Science Health (Particle Sciences) demonstrate high presence in conventional spray drying services in the American market on the pharmaceutical spray drying industry. Cambrex provides economical spray-dried dispersion development, which is the part of its drug products development.
U.S. Pharmaceutical Spray Drying CDMO Services Market Insights, By Product – Small Molecules and APIs Lead Product Demand Owing to Extensive Pharmaceutical Applications
The majority market share is occupied by small molecule and APIs with an estimated market share of 42.1% in 2025. Spray drying of small molecules and APIs is mostly demanded in the market due to their high usage in a wide range of therapeutic indicators such as cardiovascular, oncology, central nervous system, and anti-infective drugs.
Other companies like Bristol Myers Squibb, Eli Lilly, and Pfizer apply spray drying to promote the solubility and properties of small-molecule drugs in oncology, metabolic and anti-infective portfolios. Such CDMOs as Lonza Bend and Cambrex can meet this need by providing specialized spray-dried API formulation services to U.S. pharmaceutical developers.
U.S. Pharmaceutical Spray Drying CDMO Services market Insights, By Operational Scale – Pilot/Clinical-Scale Services’ Leadership is Attributable to Innovation Focus and Risk Mitigation
The pilot/clinical-scale services are likely to dominate the U.S. pharmaceutical spray drying CDMO services market with a share of 78.5% in 2025, because the pharmaceutical industry has long been dedicated to innovation, development of early-stage drugs, and mitigation measures against risks.
The examples of the U.S. players demonstrate the superiority of pilot and clinical-scale spray drying. Kineticos Life Sciences has programs that assist with early-stage work at 3 batches of rapid feasibility, and Pharmaceutic Labs offers pilot-scale manufacturing of heat-sensitive APIs to de-risk the scale-up.
Regulatory & U.S. FDA Compliance Comparison for Inhalation vs Oral Solid Dosage Formats
- In the U.S. pharmaceutical spray drying CDMO services market, inhalation products face stricter U.S. FDA controls than oral solid dosages (OSD). Inhalation powders must meet tight specifications for particle size, lung deposition, residual solvents, and microbial limits, and must comply with OINDP and device-related requirements, including extractables/leachables and 21 CFR Part 820 for device components.
- Spray-dried OSD formats follow standard cGMP and ICH guidelines, with regulatory focus on dissolution, bioavailability, stability of amorphous dispersions, and content uniformity. While both require full validation of the spray-drying process, inhalation formats carry higher compliance burden due to direct lung exposure and device drug integration.
Competitive Benchmarking: U.S. Pharmaceutical Spray Drying CDMO Services Market
Facility Expansions
- CDMOs are adding larger spray dryers to meet late-stage and commercial demand. Lonza, Expanded high-capacity spray-drying suites and HPAPI containment in bend.
- High-potency containment suites are expanding to support oncology pipelines.
- Integrated formulation–analytics hubs are rising to cut tech-transfer time.
- Expansions cluster in the Midwest/South to streamline logistics and regulatory handling.
GMP Capabilities
- Top players run fully validated GMP suites with automated CIP/SIP.
- Strong high-potency handling (OEB 4–5) is a core differentiator.
- Mature Quality Systems with ICH-aligned stability programs attract sponsors.
- Electronic batch records and audit-ready documentation improve compliance.
- Catalent – Added large-scale spray dryers (up to commercial scale) at Winchester, KY.
- Catalent Newsroom, “Catalent Completes Expansion of Kentucky Facility” (2023).
Technology Platforms
- Closed-cycle nitrogen systems enable safe solvent-rich spray drying.
- Thermo Fisher Scientific (Patheon)- Invested in additional formulation and analytical suites in Greenville & Cincinn
- Advanced particle engineering: ASDs, inhalation powders, nano-SD hybrids.
- PAT tools (NIR/Raman) optimize moisture, PSD, and residual solvents in real time.
- Seamless scale-up (bench → commercial) drives competitive advantage.
Pipeline & Clinical Development Demand Study (API Complexity Index) - U.S. Pharmaceutical Spray Drying CDMO Services Market
|
API Complexity Index Component |
Implications for U.S. Pharmaceutical Spray Drying CDMO Services Market |
|
High-Complexity APIs (Poorly Soluble NCEs) |
Drives strong demand for spray-dried dispersions (SDDs) due to solubility and bioavailability challenges. |
|
Biologics & Large Molecules Transitioning to Dry Formats |
Increases need for advanced particle engineering and low-temperature spray drying to maintain stability. |
|
Early-Stage Clinical Pipeline Expansion (Phase I–II) |
Boosts pilot/clinical-scale spray drying services as firms reduce risk before scale-up. |
|
Increase in Inhalation & Pulmonary Drug Candidates |
Raises adoption of spray drying for DPI particle-size control, aerodynamic performance, and uniformity. |
|
Complex Controlled-Release and Multi-API Formulations |
Encourages customized SDD designs and polymer–API matrix optimization capabilities. |
|
Shift Toward Orphan Drugs & Niche Indications |
Generates small-batch, high-value spray drying projects requiring precision powder processing. |
|
Regulatory Push for Quality-by-Design (QbD) |
Elevates requirement for robust spray drying process control, scalability, and validation. |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
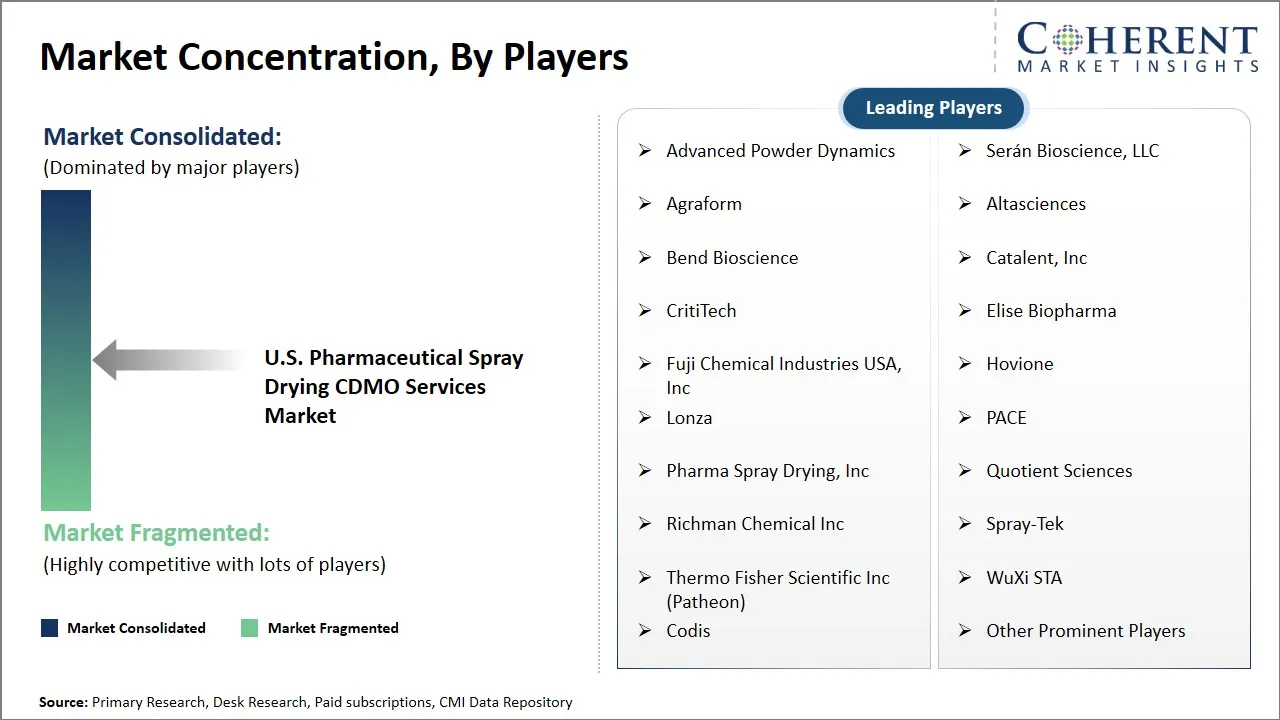
Market Players, Key Developments, and Competitive Intelligence
To learn more about this report, Download Free Sample
Key Developments
- In October 2025, Hovione, a global CDMO known for advanced spray drying, expanded its U.S. pharmaceutical spray drying capacity with a new 31,000 square foot facility in East Windsor, New Jersey. The company doubled its spray drying output after a USD 100 million investment to meet rising demand for ASD development and GMP commercial production.
- In October 2025, Evonik, a global specialty chemicals leader, began operating a new spray drying facility in Darmstadt to expand its U.S. pharmaceutical spray drying relevance through stronger excipient supply. The new plant boosts output of EUDRAGIT oral excipients and supports rising demand for advanced drug delivery. Evonik strengthened supply security by integrating the unit into its production network and improving delivery times for global customers.
- In October 2025, Codis, a new global CDMO specializing in commercial spray drying, launched after combining Particle Dynamics in the U.S. and the former EUROAPI site in Haverhill, U.K. Codis positions itself as a major player in pharmaceutical spray drying CDMO services with 40,000 square meters of capacity across the U.S. and the U.K. The company offers large-scale spray drying, amorphous solid dispersions, and particle engineering supported by 30 years of regulatory strength.
- In June 2025, Bend Bioscience, a drug formulation and particle engineering company, highlighted new progress in pharmaceutical spray drying CDMO services by exploring how spray dried dispersions can support controlled release designs. The team explained that most new drugs show poor solubility and need amorphous solid dispersions for higher bioavailability.
Top Strategies Followed by U.S. Pharmaceutical Spray Drying CDMO Services Market Players
- Established market players who are usually large multinational firms invest intensively in research and development to create innovation in high-performance spray drying items. These players are interested in developing superior formulations, which enhance drug stability, bioavailability, and efficiency of processes, thus solving complicated drug issues. They also have strategic alliances and partnerships with other key players in the industry or original equipment manufacturers (OEMs) in order to consolidate their presence in the market.
- In May 2023, Experic expanded its U.S. pharmaceutical spray drying capabilities to strengthen formulation and development services. Experic is a CDMO based in New Jersey that supports clinical and commercial manufacturing. The company added a pilot-scale spray dryer to improve solubility, control particle design and support both oral and inhalation products.
- Mid-level players in the pharmaceutical spray drying CDMO services market in the U.S. place strategic emphasis on cost-effective interventions that can offer quality without being prohibitively expensive to fulfill the needs of market segments that are price-sensitive like generic drug manufacturers, and small pharmaceutical companies. These firms focus on scalable and efficient production processes that minimize operational expenses without affecting the quality of their products and thus their products are appealing to customers with strict budget limits.
- Firms such as Bend Bioscience, Agilex Biolabs, and Ascendia Pharmaceuticals fit this profile. Bend Bioscience focuses on cost-efficient spray-dried dispersions for smaller pharma pipelines. Ascendia develops ASD solutions targeting generic and reformulation programs.
- Small-scale players use a other approach, and specialize in niche markets, either by creating very specific sprays drying solutions or novel forms of products customized to particular pharmaceutical applications. Such smaller businesses tend to focus on responsiveness and innovation and quickly turn to new technologies like continuous spray drying systems and highly sophisticated particle engineering methods to remain competitive.
- Companies such as Pharmatech Laboratories, Fluid Air (Spray Drying R&D Unit), and Biopharma Group (U.S. applications labs) represent smaller, innovation-driven entities. They specialize in niche technologies including continuous spray drying, micro-particle tailoring, and bespoke formulation platforms for early-stage molecules.
Market Report Scope
U.S. Pharmaceutical Spray Drying CDMO Services Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 790.2 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.9% | 2032 Value Projection: | USD 1,345.5 Mn |
| Segments covered: |
|
||
| Companies covered: |
Advanced Powder Dynamics, Serán Bioscience, LLC, Agraform, Altasciences, Bend Bioscience, Catalent, Inc, CritiTech, Elise Biopharma, Fuji Chemical Industries USA, Inc, Hovione, Lonza, PACE, Pharma Spray Drying, Inc, Quotient Sciences, Richman Chemical Inc, Spray-Tek, Thermo Fisher Scientific Inc (Patheon), WuXi STA, Codis, and Other Prominent Players |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
U.S. Pharmaceutical Spray Drying CDMO Services Market Dynamics
To learn more about this report, Download Free Sample
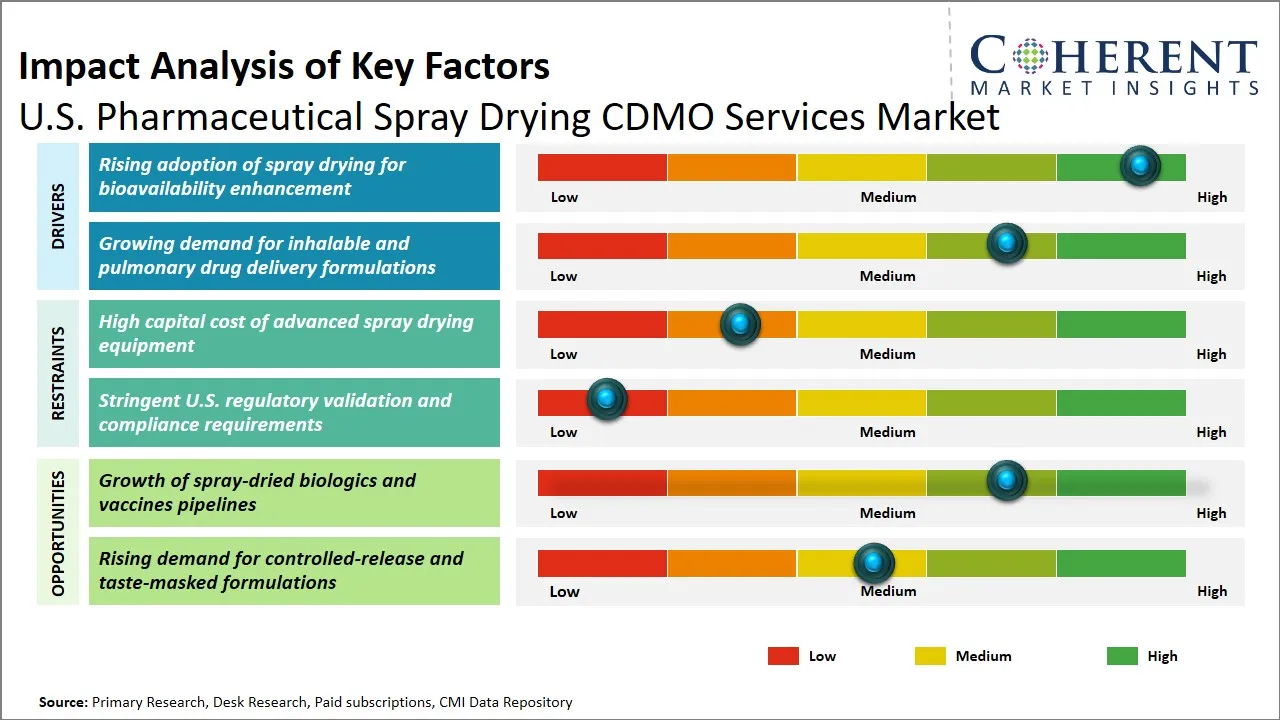
U.S. Pharmaceutical Spray Drying CDMO Services Market Driver - Rising Adoption of Spray Drying for Bioavailability Enhancement
Rise in the use of spray drying technology in the pharmaceutical sector can be greatly attributed to the fact that spray drying technology is very useful in increasing the bioavailability of the active pharmaceutical ingredient (API), especially those that are not soluble in water. Spray drying will also allow the conversion of drugs into amorphous solid dispersions or microparticles and this could dramatically enhance the dissolution rates and absorption in the human body. The technique enables the accurate regulation of the particle size, morphology, and surface properties, which guarantee enhanced drug delivery and the uniformity of therapeutic outcomes.
For example, companies such as Ethris, CEPI (via funded development partnerships), and Rockwell Automation (via process-digitization collaborations with Lonza) demonstrate how spray drying is being applied to improve bioavailability and stabilize complex RNA and biologic formulations. Ethris uses spray drying to create room-temperature stable mRNA vaccines designed for mucosal delivery, highlighting the technology’s ability to protect fragile RNA molecules while improving deliverability
U.S. Pharmaceutical Spray Drying CDMO Services Market Opportunity - Growth of Spray-Dried Biologics and Vaccines Pipelines
The U.S. pharmaceutical spray drying CDMO services market is experiencing a huge opportunity due to the growing pipeline of spray-dried biologic and vaccines. Spray drying technology has provided superior stability, better bioavailability, and administration ease, thus this is the application of choice in the sensitive biologics like proteins, peptides and vaccines.
The increasing incidence of chronic illnesses and the immediate necessity of effective vaccinations, especially, which were magnified due to the COVID-19 pandemic, have increased the pace of research and development in the area of spray-dried formulations. This is where biotech and pharmaceutical firms are putting a lot of money to solve the conventional problem associated with cold chain storage and distribution because spray-dried powdered items usually have a higher thermal stability and extended shelf lives.
Indicatively, firms like AstraZeneca, Merck & Co. and Johnson and Johnson are developing spray-dried biologic and vaccine platforms in order to enhance stability and broadening the non-injectable routes of delivery. New companies such as VaxForm and BlueWillow Biologics are coming up with intranasal and dry-powder vaccine candidates in respiratory illnesses. Catalent and Patheon, CDMOs managed by Thermo Fisher, are expanding the capacity of spray-drying operations to accommodate the increasing U.S. biologics and vaccine programs.
Analyst Opinion (Expert Opinion)
- The U.S. pharmaceutical spray drying CDMO services market is booming as pharmaceutical makers seek consistent, scaled-up formulations of biologics, vaccines and inhalable treatments. The growth in the markets is being enhanced by the development of spray drying systems, increasing interest in cold-chain-independent products, and favorable regulatory involvement despite the ongoing problem of high capital requirements and complicated biologic stability. Technical discourses are still being influenced by industry events, including AAPS PharmSci 360, CPhI North America and INTERPHEX, which are enhancing the rate of technology adoption.
- An indication of this apparent shift to more flexible and efficient manufacturing strategies can be characterized by recent efforts of biopharma companies and CDMOs, such as increased capacity of spray drying and new programs in dry-powder vaccines. These initiatives are leading to more intensive clinical development of spray-dried formulations and strengthening the use of the technology in the next-generation drug delivery approaches.
Market Segmentation
- Service Offerings Insights (Revenue, USD Mn, 2020 - 2032)
- Standard Spray Drying Services
- Formulation Development + Spray Drying
- Spray Dried Dispersion (SDD) Services
- Others Services (Scale Up & Tech Transfer & Others)
- Product Insights (Revenue, USD Mn, 2020 - 2032)
- Small Molecules and APIs
- High Potent Active Pharmaceutical Ingredients (HPAPI)
- Excipients
- Co-Spray Drying/Composite Particle Formation (API + Excipient)
- Controlled Substances
- Biologics/Proteins/Peptide
- Others (Nutraceutical/Dietary Supplement Powders and Others)
- Operational Scale Insights (Revenue, USD Mn, 2020 - 2032)
- Pilot/Clinical-Scale Services
- Commercial/Industrial-Scale Services
- Application Area Insights (Revenue, USD Mn, 2020 - 2032)
- Oral Dosage Forms (powders, granules, or ASD for tablets/capsules)
- Injectable/Parenteral (intermediate powders for reconstitution)
- Pulmonary/Inhalation Delivery (dry powders for inhalers)
- Encapsulated Formulations/Co-Spray Dried Particles
- Others (Controlled Release/Sustained Release and Others)
- End User Insights (Revenue, USD Mn, 2020 - 2032)
- Pharmaceutical Companies
- Biopharmaceutical/Biotechnology Companies
- Academic or Contract Research Organizations (CROs)
- Others (Nutraceutical and Dietary Supplement Companies and Others)
- Key Players Insights
- Advanced Powder Dynamics
- Serán Bioscience, LLC
- Agraform
- Altasciences
- Bend Bioscience
- Catalent, Inc
- CritiTech
- Elise Biopharma
- Fuji Chemical Industries USA, Inc
- Hovione
- Lonza
- PACE
- Pharma Spray Drying, Inc
- Quotient Sciences
- Richman Chemical Inc
- Spray-Tek
- Thermo Fisher Scientific Inc (Patheon)
- WuXi STA
- Codis
- Other Prominent Players
Sources
Primary Research Interviews
Industry Stakeholders list
- Formulation scientists at U.S.-based pharmaceutical CDMOs
- Process development heads at large U.S. pharma manufacturers
- Manufacturing/site operations managers overseeing spray drying units
- Quality & regulatory affairs managers in solid dosage manufacturing
- R&D directors in oral solid dosage technology platforms
- CMC consultants specializing in spray drying
End Users List
- Clinical pharmacists using ASD-based formulations in hospitals
- Physicians prescribing ASD-based oral drugs
- Key opinion leaders in drug delivery and formulation science
- Procurement managers at U.S. pharma and biotech companies
- Supply chain managers at pharmaceutical manufacturers
- Technology transfer leads at biopharma firms
Government and International Databases
- FDA – Drugs@FDA
- FDA Inactive Ingredient Database (IID)
- U.S. National Library of Medicine – DailyMed
- ClinicalTrials.gov
- EMA – EPAR database
- WHO technical reports
Trade Publications
- Pharmaceutical Technology
- Pharma Manufacturing
- Pharmaceutical Online
- Contract Pharma
- European Pharmaceutical Review
- Manufacturing Chemist
Academic Journals
- International Journal of Pharmaceutics
- European Journal of Pharmaceutics and Biopharmaceutics
- Journal of Pharmaceutical Sciences
- AAPS PharmSciTech
- Drug Development and Industrial Pharmacy
- Journal of Drug Delivery Science and Technology
Reputable Newspapers
- The Wall Street Journal
- The New York Times
- Financial Times
- The Washington Post
- The Guardian
- Reuters
Industry Associations
- International Society for Pharmaceutical Engineering (ISPE)
- Parenteral Drug Association (PDA)
- American Association of Pharmaceutical Scientists (AAPS)
- Biotechnology Innovation Organization (BIO)
- Pharmaceutical Research and Manufacturers of America (PhRMA)
- Controlled Release Society (CRS)
Public Domain Resources
- USPTO patent database
- Espacenet (European Patent Office)
- U.S. Census Bureau
- OECD health and pharmaceutical statistics
- NIH publications repository
- U.S. Bureau of Labor Statistics (BLS)
Proprietary Elements
- CMI Data Analytics Tool: Proprietary analytics tool to analyze real-time market trends, consumer behavior, and technology adoption in market
- Proprietary CMI Existing Repository of Information for Last 8 Years
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients