Global acute kidney injury treatment market is estimated to be valued at USD 1.96 Bn in 2025 and is expected to reach USD 3.43 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 8.3% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
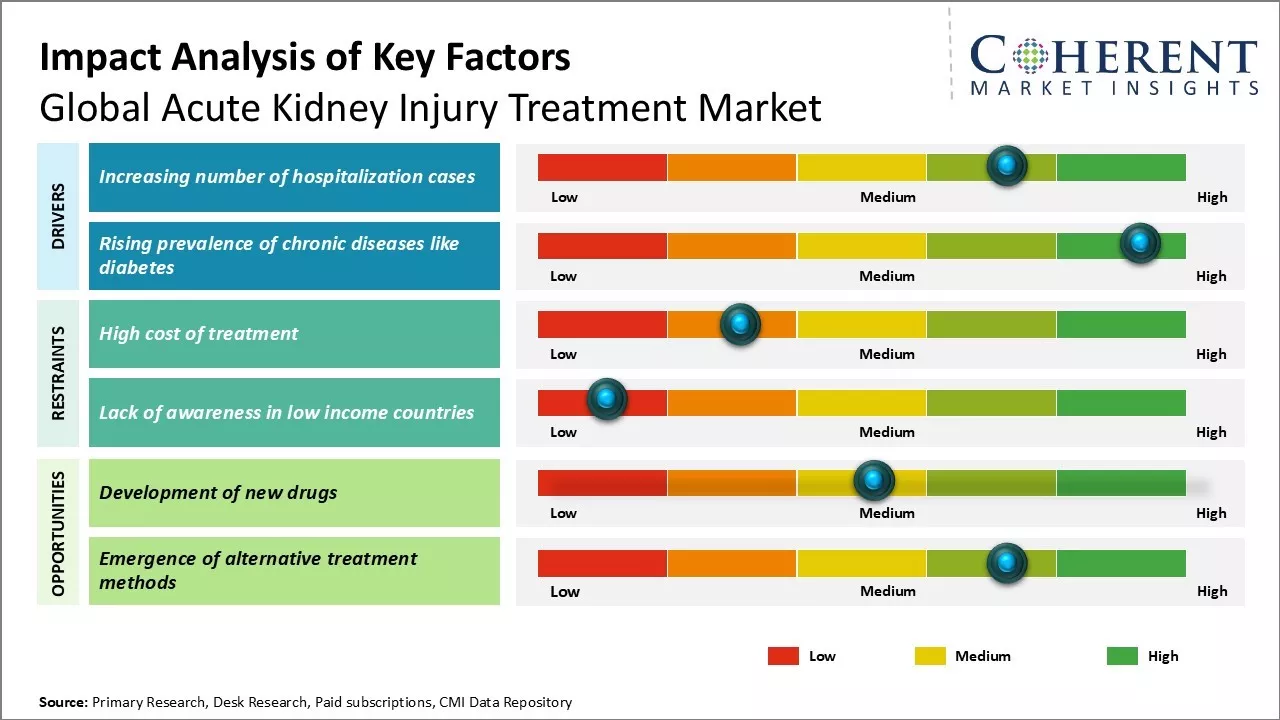
Increasing risk factors associated with acute kidney injury such as increasing aging population, growing incidence of diabetes and hypertension, and rising number of surgeries can drive the market growth. Moreover, development and approval of newer drugs for acute kidney injury treatment along with availability of advanced dialysis technologies can also drive the market growth. However, lack of awareness about acute kidney diseases especially in developing nations and high cost of treatment procedures can hamper the market growth. Increasing research activities and stringent regulatory guidelines to minimize drug-induced kidney injuries can offer lucrative opportunities for the acute kidney injury treatment market players over the forecast period.
Increasing number of hospitalization cases
With growing prevalence of various chronic conditions and lifestyle diseases across the world, there has been increase in number of hospital admissions over the past few decades.
Prolonged hospital stays often necessitate the use of potent medications, intravenous contrast agents, and other invasive procedures, which can overburden the kidneys and impair their normal functioning. The hospital environment also increases the risk of acquiring infections like sepsis, which is one of the leading causes of acute kidney injuries. Moreover, major surgeries, especially those involving the cardiovascular or gastrointestinal systems, place heavy stress on the kidneys. As global disease burden increases, more complex procedures are being performed to manage advanced cases that further elevates the risk. Therefore, with continually swelling patient volumes inside hospitals and longer average hospitalization times, patients are more susceptible to acute renal complications.
Get actionable strategies to beat competition: Download Free Sample
Rising burden of chronic diseases like diabetes
Chronic medical conditions have emerged as the foremost challenge for healthcare systems worldwide. Rising prevalence of lifestyle-related long-term disorders especially diabetes and hypertension is the major trend in the market. These non-communicable diseases have turned into silent epidemics engulfing both developed and developing populations alike. Both diabetes and high blood pressure are significant risk factors for kidney diseases as these can cause steady damage to the renal structures over extended periods of time, if not properly managed.
As more people live with diabetes and hypertension for many years without control, the risk of eventually developing chronic kidney disease and end-stage renal failure has multiplied substantially. Other chronic conditions like autoimmune diseases and glomerulonephritis are also linked to long-term renal impairment. Ageing global demographics with more elderly susceptible to co-morbidities further exacerbates the chronic disease dynamics. As populations suffer from metabolic, cardiovascular and other long-standing illnesses, their odds of acquiring acute renal problems superimposed upon crumbled kidney functions climbs up considerably.
Key Takeaways from Analyst:
Global acute kidney injury treatment market growth is driven by rising prevalence of diabetes and hypertension, which are major risk factors for AKI. Increasing risk of AKI among the rapidly growing aging population can also drive the market growth. However, lack of approved drugs and limited treatment alternatives can hamper the market growth.
North America currently dominates the market due to high healthcare spending and growing incidence of AKI. However, Asia Pacific is expected to emerge as the fastest growing region due to large patient pool, rising disease prevalence, and improving access to healthcare in developing countries. The market also witness notable opportunities in terms of R&D into novel drug therapies and combination treatment options. Major companies are focusing on clinical trials for pipeline drugs to expand their portfolios.
Dialysis will continue dominating the treatment landscape over the forecast period. However, there has been huge demand for alternative treatment such as therapeutic interventions targeting underlying disease pathways. Strong pipeline with drugs in various clinical trial phases indicate promising growth potential through new product approvals. Challenging nature of developing drugs makes collaborations and partnerships common in this market. Increased focus on research for advanced treatment options can drive the global acute kidney injury treatment market growth.
Market Challenges: High cost of treatment
Global acute kidney injury treatment market growth can be hampered due to high cost associated with the treatment of acute kidney injury. Acute kidney injury requires intensive care and frequent dialysis procedures, which can be expensive. The expensive hospital stays, costly medications and the dialysis treatment increases economic burden on patients as well as national healthcare systems. This high cost of treatment can hamper the widespread adoption of therapy and drug regimens. The out of pocket expenses for patients suffering from acute kidney injuries discourage them from opting for prescribed treatment courses. This negatively impacts the market revenues as lesser number of patients avail kidney injury care. Moreover, rising healthcare costs have increased the financial pressure on governments and private insurance companies. Controlling treatment expenses remains a key priority for market growth in the near future.
Market Opportunities: Development of New Drugs
The development of new drugs for acute kidney injury treatment can offer major opportunity for players in the global acute kidney injury treatment market. Currently, there are very few approved drug therapies for preventing or treating acute decline of kidney function. The existing therapeutic options are also associated with side effects and have limited efficacy rates. This leaves a large void that can be potentially filled by novel drug molecules. Significant research is now focused on identifying new biological targets and developing innovative drugs such as stem cell therapies, gene therapies and organ regeneration techniques. Successful clinical research campaigns leading to the approval of such new drugs can address currently unmet needs. This can aid in faster recovery, better clinical outcomes for patients and most importantly reduce overdependence on costly renal replacement therapies like dialysis. The emergence of advanced treatment alternatives ccan boost market revenues by driving new patient adoptions in the future.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
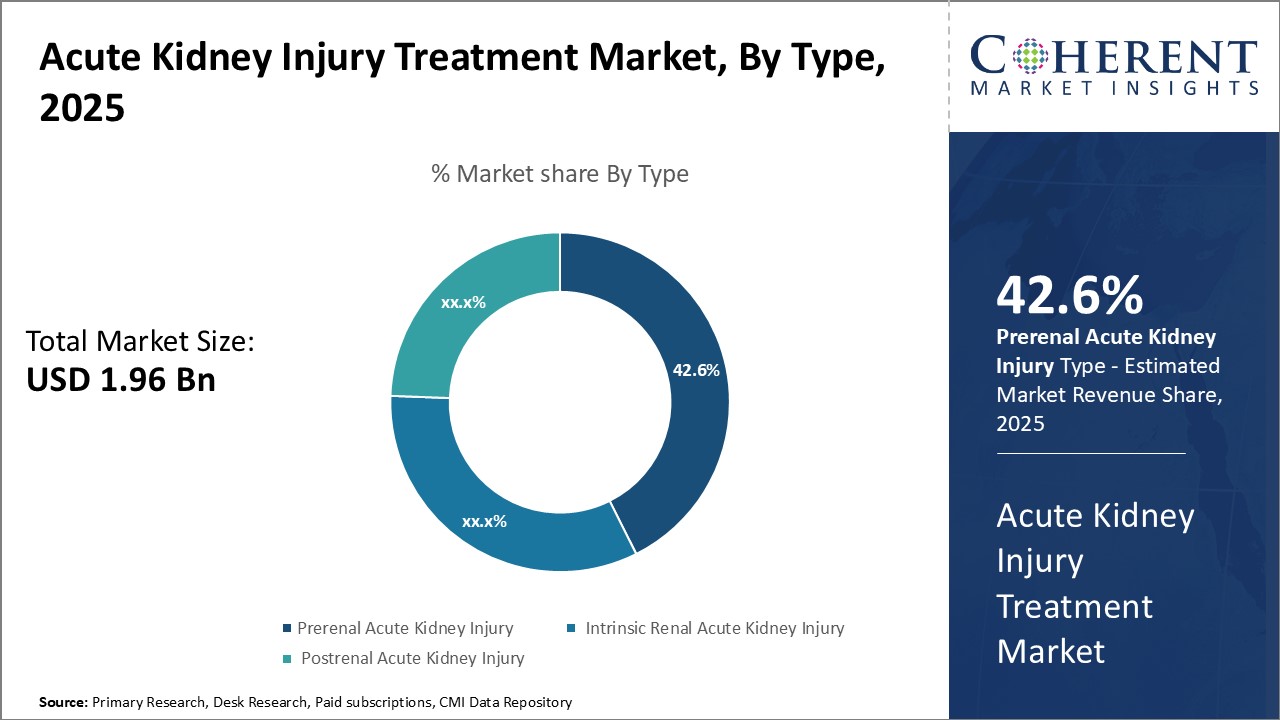
By Type- Prevalence of lifestyle diseases can drive the prerenal acute kidney injury segment
By type, prerenal acute kidney injury segment is estimated to contribute the highest market share of, owing to rising lifestyle diseases like diabetes and hypertension. Prerenal acute kidney injury, also known as pre-renal azotemia, refers to impaired renal perfusion or hypoperfusion of the kidneys due to factors prior to the kidneys. The major causes are reduced cardiac output, low blood pressure due to dehydration or bleeding or use of certain medications. With growing sedentary lifestyles and junk food consumption, cases of diabetes, hypertension and cardiovascular diseases have increased manifold in the recent years. These conditions are a major risk factor for impaired kidney perfusion leading to prerenal acute kidney injury. Moreover, factors like aging population and excessive alcohol intake have further exacerbated the risk. Since prerenal acute kidney injury has identifiable causes outside the kidneys, it is more prevalent and contributes the largest share in the total acute kidney injury treatment market.
By Treatment – Prevalence of comorbidities drives therapy segment
By treatment, therapy segment is estimated to contribute the highest market share of, owing to increasing prevalence of comorbid medical conditions in acute kidney injury patients. Therapy aims to improve renal perfusion and correct the underlying causes of impaired kidney function. It includes measures like fluid resuscitation, vasopressor support, relief of obstructive uropathy and renal replacement therapy as needed. Most acute kidney injury cases are associated with comorbidities like sepsis, major surgery, trauma, and others. Managing these concurrent diseases and conditions assumes high priority even as renal recovery is promoted simultaneously. Given its capacity for holistic management of both renal and non-renal illnesses, therapy forms the cornerstone of acute kidney injury treatment and attracts greater demand over drug-based approaches.
By Distribution Channel -Higher diagnostic accuracy drives hospital pharmacies segment
In terms of distribution channel, hospital pharmacies segment is estimated to contribute the highest market share of, owing to more accurate diagnosis and timely treatment availability. Hospital pharmacies have several locational and accessibility advantages for acute kidney injury patients. These are generally the initial points of medical contact during emergencies. Investigations for diagnosing the underlying cause can be carried out rapidly in a hospital set-up. Specialized nephrology services as well as intensive care facilities are well-established in most hospitals to reverse acute kidney injury. This ensures higher diagnostic accuracy and quicker intervention. Hospital pharmacies also stock a wider range of acute kidney injury drugs and are well-equipped to support therapy regimens seamlessly. With greater reliability on diagnosis and treatment outcome, hospital pharmacies account for the highest share in the market.
Need a Different Region or Segment? Download Free Sample
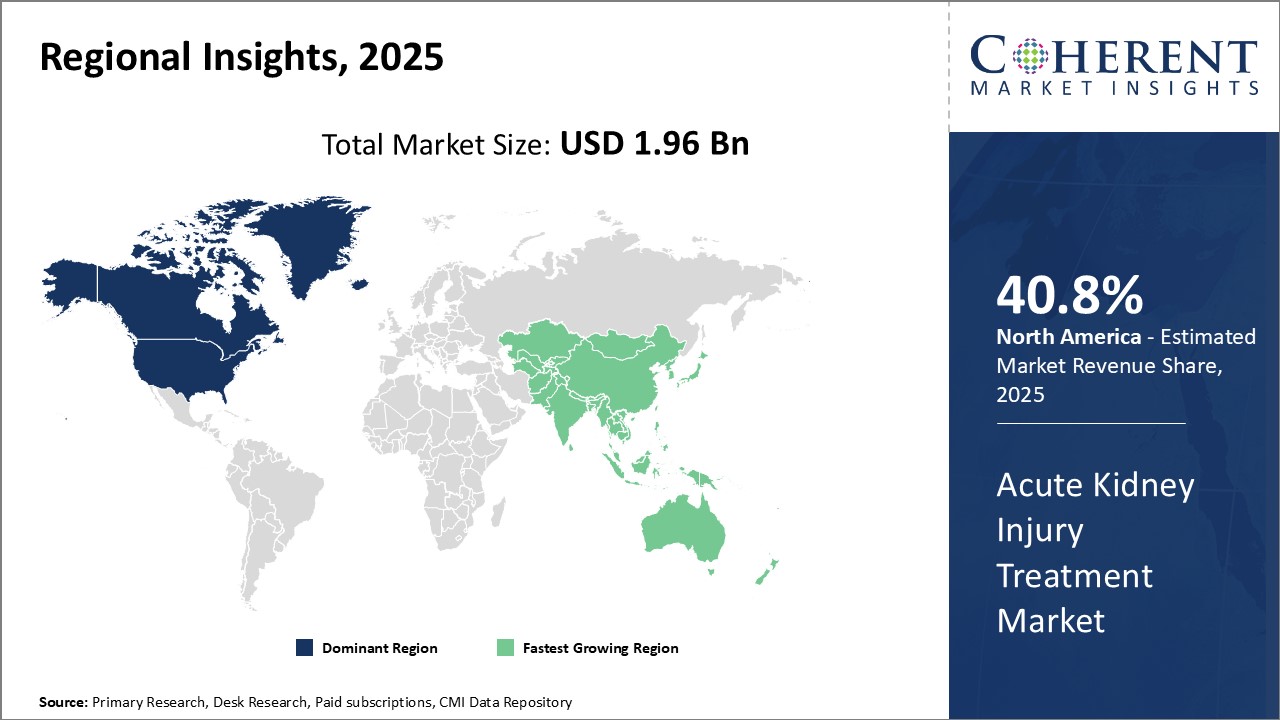
North America currently dominates the global acute kidney injury treatment market, with an estimated market share of 40.8% in 2025, owing to growing incidence of kidney diseases, advanced healthcare facilities and increased adoption of novel therapeutics. The U.S. contributes significantly to acute kidney injury treatment needs of the region due to rising healthcare spending and presence of leading industry players.
Asia Pacific excluding Japan represents the fastest growing regional market due to expanding patient pool, improving access to healthcare, and growing medical tourism across countries like India and China. Several factors are contributing to increased demand for acute kidney injury treatment in the region including rapidly aging population leading to higher prevalence of comorbidities, evolving lifestyle and dietary habits increasing the risk of renal disorders. Furthermore, favorable regulatory guidelines and growing focus of key players on Asia Pacific markets seeking new avenues for growth can drive the market growth. Government efforts to promote generic and biosimilar production in India and China have improved the accessibility and affordability of acute kidney injury drugs in the region.
The regulatory landscape is also relatively simplified in many Asia Pacific countries to attract global investments. This has prompted global pharma companies to strengthen their presence through opening manufacturing facilities, collaborating with local pharma players or through acquisitions. While developed markets like Japan contribute a fair share currently, overall Asia Pacific market propelled by high population nations.
Acute Kidney Injury Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.96 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.3% | 2032 Value Projection: | USD 3.43 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
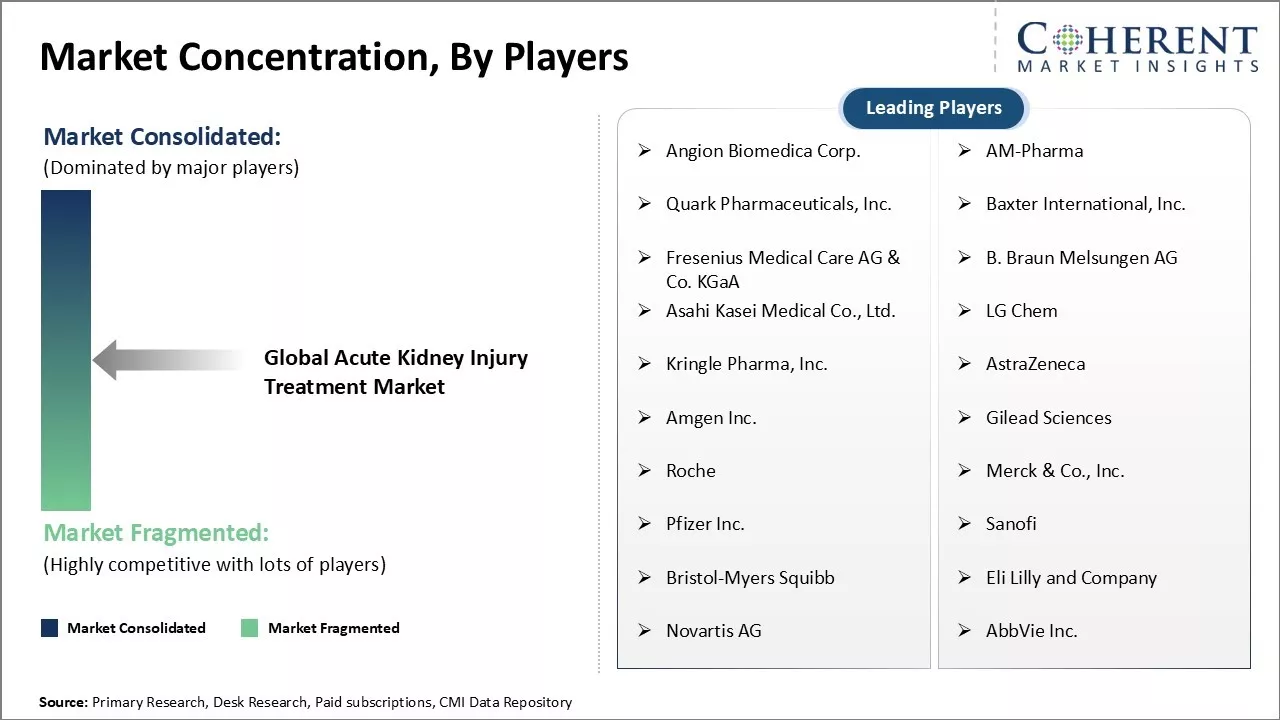
Angion Biomedica Corp., AM-Pharma, Quark Pharmaceuticals, Inc., Baxter International, Inc., Fresenius Medical Care AG & Co. KGaA, B. Braun Melsungen AG, Asahi Kasei Medical Co., Ltd., LG Chem, Kringle Pharma, Inc., AstraZeneca, Amgen Inc., Gilead Sciences, Roche, Merck & Co., Inc., Pfizer Inc., Sanofi, Bristol-Myers Squibb, Eli Lilly and Company, Novartis AG, and AbbVie Inc |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients