Plasmid DNA is becoming increasingly important in clinical research for gene therapy and genetic vaccine applications. Good manufacturing practice (GMP)-grade plasmid DNA is required for direct gene transfer into humans. The same is true if the drug material contains a genetically modified cell, such as chimeric antigen receptor (CAR) T cells, which are employed along with the plasmids present in the drug substance.
In many circumstances, however, high quality grade plasmid DNA is approved as a starting material for GMP synthesis of mRNA or viral vectors (lentiviral vectors, adeno-associated virus vectors, and so on). AAV (adeno associated viruses) vectors support the production of protein, leading to therapeutic effect, without permanently altering the patient’s DNA. Lentiviral vectors are retroviruses used to introduce a functional copy of a gene to the patient’s extracted hematopoietic stem cells with a longer-term therapeutic effect.
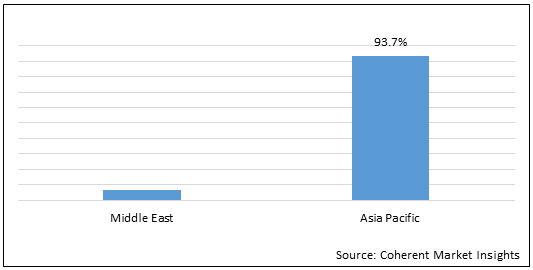
Middle East and Asia Pacific cell and gene therapy market is estimated to be valued at US$ 2,616.8 million in 2021 and is expected to exhibit a CAGR of 17.3% over the forecast period (2021-2028).
Figure 1. Middle East and Asia Pacific Cell and Gene Therapy Market Share (%), in Terms of Value, By Region, 2021
To learn more about this report, Download Free Sample
Increasing launch and approval of novel gene therapies for the treatment of various rare diseases is expected to derive the market growth
In June 2017, Horama S.A. a biotechnology company announced that U.S. Food and Drug Administration (FDA) granted Orphan Drug Designation (ODD) to HORARLBP1. A gene replacement therapy Moreover, HORARLBP1 is developed for the treatment of retinitis punctata albescens and retinitis pigmentosa caused by mutations in the RLBP1 gene.
Middle East and Asia Pacific Cell and Gene Therapy Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2020 | Market Size in 2021: | US$ 2,616.8 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2021 to 2028 |
| Forecast Period 2021 to 2028 CAGR: | 17.3% | 2028 Value Projection: | US$ 8,015.1 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Novartis International AG, Pfizer, Inc., Sanofi S.A., Amgen, Inc., Regeneron Pharmaceuticals, Inc., F. Hoffmann-La Roche AG, Bluebird Bio, Inc. (Celgene Corporation), Gene biotherapeutics, Sibiono GeneTech Co. Ltd., Kolon TissueGene, Inc., Horama S.A., MeiraGTx Limited, Gilead Sciences, Inc., Biogen INC., Organogenesis, Inc., JCR Pharmaceuticals Co. Ltd, uniQure N.V., WuxiAppTec, Lonza, and Immuneel Therapeutics Pvt Ltd |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
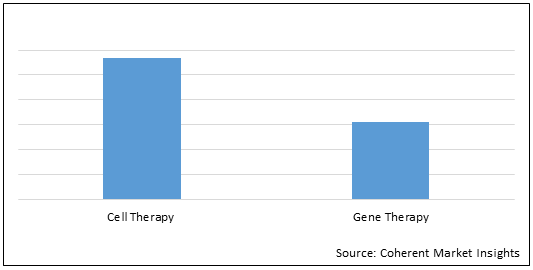
Figure 2. Middle East and Asia Pacific Cell and Gene Therapy Market Share (%), by Therapy Type, 2021
To learn more about this report, Download Free Sample
Middle East and Asia Pacific Cell and Gene Therapy Market – Impact of Coronavirus (COVID-19) Pandemic – Impact on Drug Development and Clinical Trials
While the healthcare industry continues to focus on the development of COVID-19 vaccines and therapies, the COVID-19 crisis had a significant impact on clinical trials in many therapy areas such as oncology, cardiovascular, and others.
Research programs and preclinical operations have been affected across the cell and gene therapies (CGTs) sector, as pharmaceutical businesses have restricted the number of individuals working on-site to keep them safe and comply with regulatory instructions.
The increasing demand for COVID-19-related laboratory consumables such as personal protective equipment and reagents has hampered the research and preclinical development, resulting in supply chain constriction.
Cell and gene therapies (CGTs) are at the forefront of medical advancements in the treatment of serious diseases such as cancer, cardiovascular diseases, and others. Many more CGTs are currently being developed.
Middle East and Asia Pacific Cell and Gene Therapy Market: Restraint
Factor such as unfavorable reimbursement policies for cell and gene therapy products is expected to hamper the market growth over the forecast period.
Kymriah is a genetically-modified autologous T-cell immunotherapy has listed price of US$ 475,000 for patients with acute lymphocytic leukemia and US$ 373,000 for patients with large B-cell lymphoma; whereas, Yescarta has a listed price of US$ 373,000. Indicated for the treatment of adults with follicular lymphoma or certain types of large B-cell lymphoma Therefore, treatment with such a high price requires government support, in terms of reimbursement policy for a better adoption rate.
However, cell and gene therapy has witnessed multiple setbacks from regulatory authorities in charge of reimbursement policies, which is expected to hinder growth of the market.
Key Players
Major players operating in the Middle East and Asia Pacific cell and gene therapy market include Novartis International AG, Pfizer, Inc., Sanofi S.A., Amgen, Inc., Regeneron Pharmaceuticals, Inc., F. Hoffmann-La Roche AG, Bluebird Bio, Inc. (Celgene Corporation), Gene biotherapeutics, Sibiono GeneTech Co. Ltd., Kolon TissueGene, Inc., Horama S.A., MeiraGTx Limited, Gilead Sciences, Inc., Biogen INC., Organogenesis, Inc., JCR Pharmaceuticals Co. Ltd, uniQure N.V., WuxiAppTec, Lonza, and Immuneel Therapeutics Pvt Ltd.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients