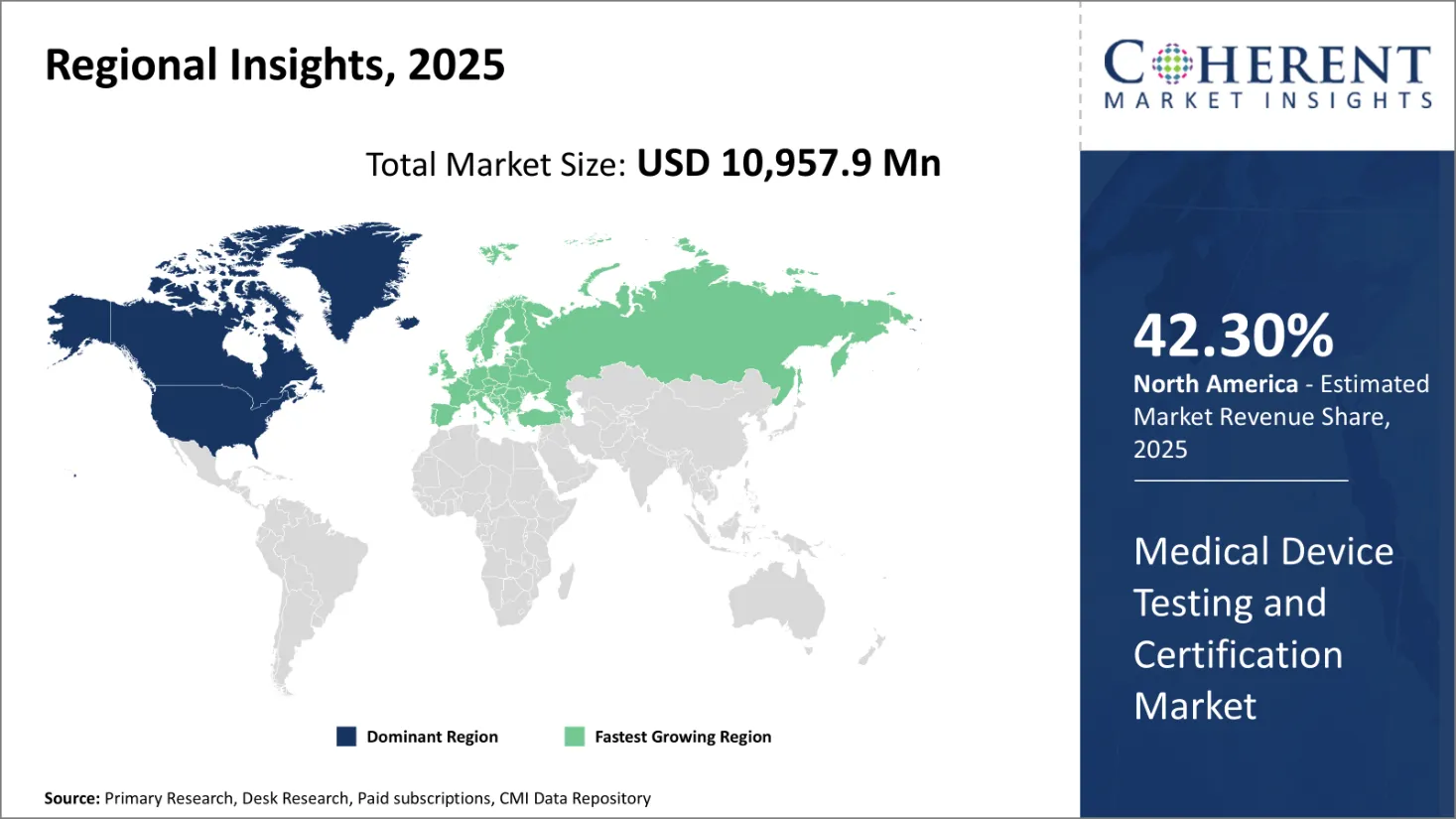
Medical Device Testing and Certification Market is poised to hold a valuation of USD 10,957.9 Mn in 2025 and is expected to reach USD 14,713.6 Mn in 2032. The market is expected to exhibit a CAGR of 4.3% from 2025 to 2032.
Various rules regarding the use of medical equipment to keep people safe all around the world. These rules are getting strict because medical devices are becoming more complicated, and medical institutions need to make sure they are safe for patients. Various measures such as new technology and safety problems with devices are pushing the need for better testing solutions. Since medical equipment now often has computers and software inside, we need to test them carefully to make sure they work well and meet the rules. Testing services are really important because they check if devices are safe, work correctly, and don’t cause harm. High investment is being made on healthcare, and countries are making rules that match up, so there’s a high need for devices to be tested. New tools, like smart AI testing and digital platforms, make it easier to test medical devices in all parts of the world, whether in rich or developing countries.
For example, in 2024, a company named Tüv Süd made a new system that lets them check medical equipment online in real-time to make the testing faster and help companies follow the rules better.
|
Current Event |
Description and its impact |
|
Increased Global Regulatory Stringency |
|
|
Advancements in Digital Health and Software-as-a-Medical-Device (SaMD) |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
A key driver of growth in the medical device testing and certification market during the forecast period is the increasing emphasis on comprehensive testing and verification of medical equipment.
In January 2022, TÜV SÜD announced plans to expand its Medical & Health Services (MHS) facilities in New Brighton, Minnesota. The 20,000 square foot laboratory extension of its existing 36,000 square foot test facility was scheduled for completion by June 2022. This expansion aimed to offer end-to-end testing solutions for all medical devices, including a wide range of biological and chemical testing services, thereby enhancing the company's capabilities in active medical device testing.
Implementation of strict regulatory standards globally is another driver that supports growth in the market. Medical equipment rules vary across the country, and manufacturers must follow these standards to sell their products in a specific area. For example, the United States follows the guidelines prescribed by the Food and Drug Administration (FDA), in the European market according to Europe in Canada, Health Canada's approval controls, and Central Drug's Standard Control Organization (CDSCO) controls medical equipment approval in India. Compliance with this regulatory structure ensures product safety and quality, which means that the need for certified test services strengthens.
For instance, In May 2023, The new microbiology and chemistry laboratory in New Brighton was accredited under ISO/IEC 17025:2017 by A2LA. This accreditation confirms the laboratory's competence in conducting biological and chemical testing of paediatric medical devices, including microbiology, reusable device testing, chemistry, biocompatibility, and packaging. The facility was constructed following Minnesota state guidelines for energy efficiency and sustainable building practices.
Increasing usage of advanced technology such as artificial intelligence (AI) and Internet of Things (IoT) will provide an opportunity for growth. The integration of these advanced technologies in real -time patient monitoring applications, has added complexity to the medical composite equipment landscape. In order to obtain marketing approval, these units will have to undergo a complete test and follow the strict regulatory requirements. Therefore, manufacturers make significant investments in test procedures to meet regulatory standards and achieve market authority.
By Service Type, Testing Services Division is expected to remain the dominant segment throughout the forecast period, driven by the growing need for safe and high-performing medical devices. These services ensure compliance with strict regulatory benchmarks, reducing failure risks and enhancing patient safety. The rise in medical innovations further underscores the demand for thorough and regular testing.
On the basis of Sourcing Type, Outsourced segment is projected to dominate due to their cost-effectiveness and access to specialized expertise. This trend is especially prevalent among small and medium-sized enterprises seeking to accelerate product development while managing overhead costs. In contrast, internal purchasing although offering process control holds a smaller share owing to the high costs and infrastructure complexity required to maintain regulatory compliance in-house.
By Device Class, Class I medical devices division leads the market due to their widespread use in basic healthcare and minimal regulatory challenges. Their strong presence in routine care across developing markets fuels growth.
On the basis of Technology, Active implantable medical device—such as pacemakers and neurostimulators—segment leads owing to their crucial roles in patient care and stringent regulatory requirements. The rise in chronic conditions drives sustained demand. Active medical equipment, including ventilators and infusion pumps, follows closely, especially in high-dependency care and emergency settings.
To learn more about this report, Download Free Sample
North America is estimated to have the largest market share during the forecast period. In the United States, the Food and Drug Administration (FDA) is a primary regulatory body that maintains medical equipment to ensure their safety and efficiency. The FDA also manages the national program, which aims to reduce exposure, and ensure the safe use of electronic products that emit ionization and non-or-ion radiation.
Europe and Asia Pacific are also estimated to experience medical equipment testing and a significant increase in certification markets, focus on fuel from the effect of epidemic, an elevated attention to the quality of the unit and strict regulatory standards in these areas. In Europe, the CE marking process includes various institutions such as competent officers, notified bodies and authorized representatives. While regulation of medical equipment is handled at the EU Member State level, the European Medicine Agency (EMA) plays a role in the overall regulatory landscape. When the unit has passed the necessary analogy assessment, manufacturers are allowed to attach the CE mark.
The United States has a prominent place in the global market for medical equipment testing and certification. With a strong regulatory structure led by the US Food and Drug Administration (FDA), the country maintains high standards for safety and efficiency of medical equipment. The outbreak of COVID-19, with the release of the FDA to free the FDA to streamline the further process, emphasized the outbreak of the COVID-19 need for rapid testing and certification of equipment. In addition, continuous innovation and integration of AI and IoT into the health care system has increased the complexity of medical equipment, which increases the demand for strong testing and certification processes.
Germany is an important contributor to the European Medical Equipment Test and Certification Market. As a centre for medical technology innovation, Germany's strong health care and regulatory compliance with EU MDR (medical equipment) ensures high quality standards. The country's cooperation with notified bodies and commitment to mark processes strengthens the leadership of the European market.
The United Kingdom is still an important player in the European Medical Equipment Test and Certification Market. Regardless of the Braxit, the UK maintains strong regulatory practice through medicines and health product regulation agency (MHRA). The high amounts of medical equipment in public health services, in collaboration with strict monitoring and safety requirements after the market, forward the demand for certification services. The UK's active attitude towards digital health technologies and AI integration in medical equipment also improves the requirement for advanced test protocols.
Testing of medical equipment and certification of disclosure for patent landscape testing for the market, unit verification technologies and continuous increase in innovation in regulatory conformity systems. Important focus areas include performance testing, BI -Revalic evaluation, electromagnetic compatibility (EMC) and software verification - especially in light of increasing digitalisation of medical equipment and connection.
Over the past decade, there has been a significant rise in patent applications related to testing equipment and medical device technologies. This trend is largely driven by stringent global regulatory standards and the growing complexity of advanced medical devices, such as wearables, implantable, and in vitro diagnostics (IVD). Patent filings have primarily come from major medical device manufacturers, contract research organizations (CROs), and testing service providers.
The reimbursement landscape for testing and certification market for medical equipment is integrated to use commercial viability and new technologies. While reimbursement policy varies from the region and the country, the landscape is primarily run by regulatory standards, payment requirements and types of medical equipment tested or certified.
The testing and certification of medical equipment are closely tied to reimbursement frameworks and the specific types of tests required. Often, the reimbursement process can be complicated, as testing services are frequently categorized separately from the actual medical devices, leading to administrative and financial challenges for manufacturers and service providers.
Reimbursement of medical equipment is often related to the approval position. When a tool is certified or approved by the relevant regulatory body (eg FDA, CE mark or Health Canada), it is qualified for reimbursement under public or private health care programs.
When the health care system leads to value -based care and patient -focused reimbursement, interest in the reimbursement of medical equipment increases. Some new reimbursement models that may affect testing and certification market for medical equipment include:
Medical equipment manufacturers must consider several factors when reimbursement of testing and certification services:
Healthcare professionals, including doctors, doctors and medical professionals, play an important role in using and using medical equipment. Their preferences on testing and certification of medical equipment are strongly influenced by several factors that ensure that equipment meets safety, power and regulatory standards.
Healthcare providers tend to favour medical equipment that has undergone rigorous testing and certification processes. They place strong trust in devices that are tested and approved by recognized regulatory authorities such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), or Health Canada.
Healthcare providers often require robust clinical evidence demonstrating the effectiveness of medical devices before recommending them for patient use. They tend to prefer devices that have undergone comprehensive clinical trials or post-market surveillance, which confirm the device's ability to deliver positive health outcomes.
The quality of medical equipment is a top priority for healthcare providers, particularly in relation to patient safety. They favour devices that have undergone rigorous biocompatibility testing, mechanical testing, and failure mode analysis to ensure durability, reliability, and consistent performance during use.
Healthcare providers are increasingly embracing innovative medical devices that incorporate cutting-edge technologies such as Artificial Intelligence (AI), the Internet of Things (IoT), and other smart features. These advancements enhance clinical capabilities, enable real-time patient monitoring, and improve diagnostic and treatment precision.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 10,957.9 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.3% | 2032 Value Projection: | USD 14,713.6 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medistri SA, BSI Group, Ente Certificazione Macchine, GMED, IMQ Group SRL, TOXIKON, TÜV SÜD, WuXi AppTec, Pace Analytical Services LLC, Gateway Analytical LLC, Boston Analytical, Bureau Veritas, UL LLC, TUV Rheinland, SGS SA, Intertek Group PLC, Eurofins Scientific, Element Materials Technology, Dekra Testing and Certification GmbH, and Institute for testing and Certification Inc., among others. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients