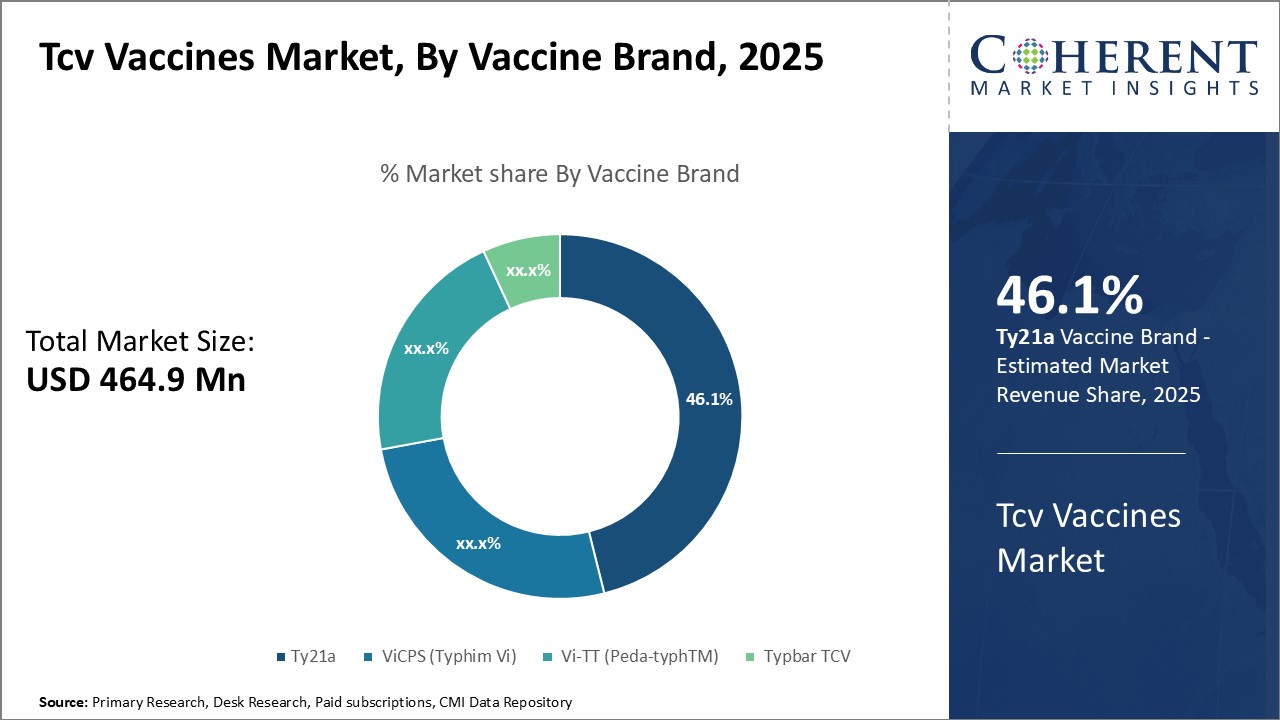
TCV vaccines market is estimated to be valued at USD 464.9 Mn in 2025 and is expected to reach USD 1,067.5 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 12.6% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
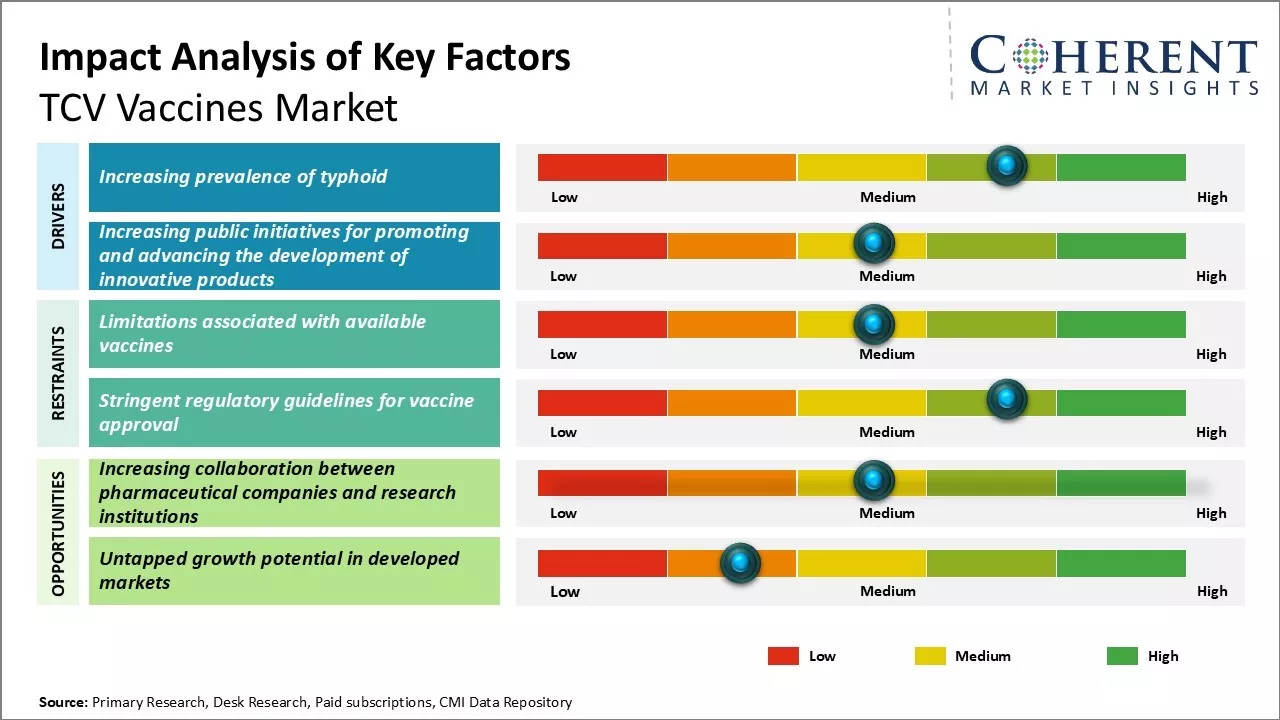
The global TCV vaccines industry is experiencing significant growth, driven by increasing initiatives from governments and non-profit organizations to vaccinate populations against typhoid fever. Key factors fueling this expansion include rising awareness of typhoid's prevalence, enhanced healthcare infrastructure, and strategic partnerships aimed at improving vaccine accessibility. However, challenges such as limited supply chains and varying regional demand can restrain growth. Despite these obstacles, the commitment to combat antibiotics resistance and the push for innovative vaccine solutions are expected to further propel market development in the coming years.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
Insights, By Vaccine Brand - Ty21a (Vivotif) Dominates Due to Strong Brand Recognition and Efficacy
In terms of vaccine brand, Ty21a (Vivotif) segment is estimated to contribute the highest market share of 46.1% in 2025 owing to its strong brand recognition and proven efficacy. As the original oral TCV vaccine, Ty21a (Vivotif) has been safely administered. Decades of use have demonstrated that it provides protective immunity comparable to injectable vaccines while avoiding needles.
Insights, By Distribution Channel- Predominate Due to Government Prioritization
In terms of distribution channel, public segment is estimated to contribute the highest market share of 67.7% in 2025. This predominance stems from the priorities of governments and international health organizations to make TCV vaccination as widely and affordably available as possible. Most countries include TCV vaccination in routine childhood immunization schedules funded through public health budgets. As a result, the United Nations International Children's Emergency Fund (UNICEF) and other aid organization tenders constitute a major portion of overall TCV distribution.
Need a Different Region or Segment? Download Free Sample
Regional Analysis: TCV Vaccines Market
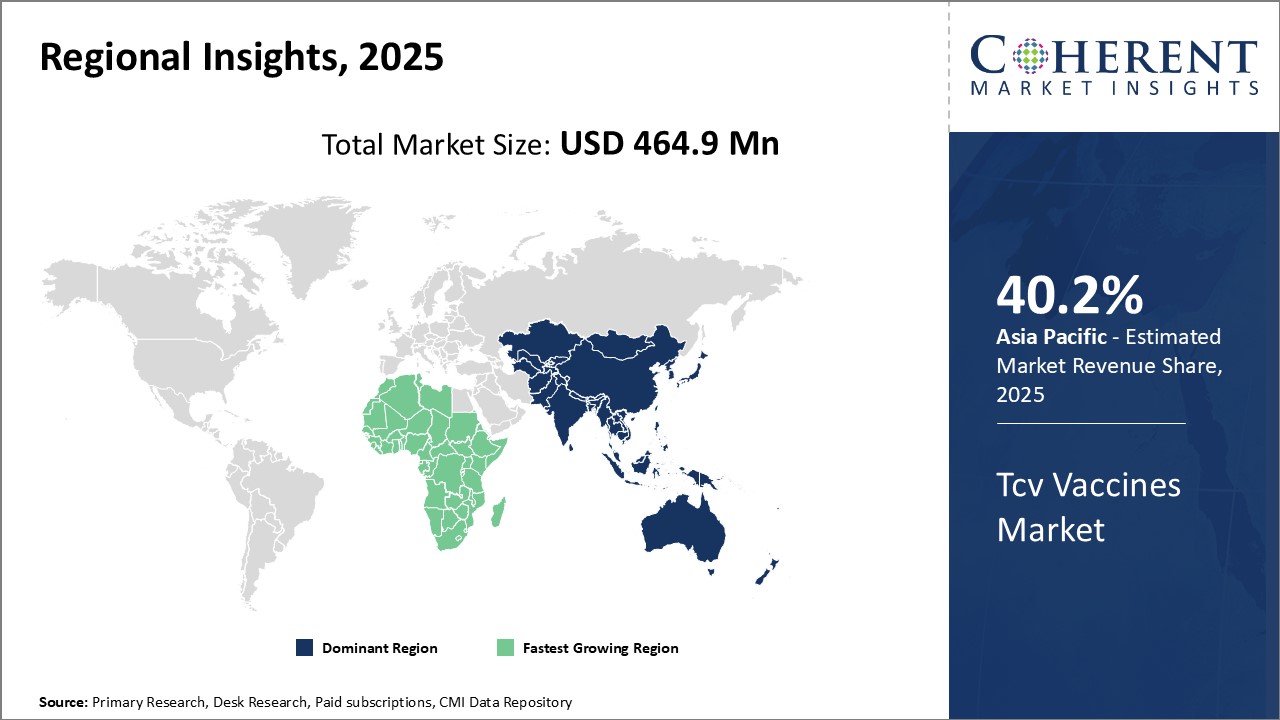
Dominating Region: Asia Pacific
Asia Pacific is expected to dominate the TCV vaccines sector with the highest market share of 40.2% in 2025. This dominance is attributed to several factors, including the high prevalence of typhoid fever, ongoing improvements in healthcare infrastructure, and increasing government initiatives aimed at enhancing immunization programs.
Fastest-Growing Region: Africa
The Africa region exhibits the fastest growth with 19.9% of the market share in 2025 in the TCV vaccines industry. This is due to the high burden of typhoid fever in many African countries, increasing government initiatives to enhance vaccination programs, and support from international organizations like Gavi and UNICEF. Additionally, the establishment of regional vaccine manufacturing capabilities and collaborative efforts to improve healthcare infrastructure further contribute to the rising demand for TCV vaccines across the continent.
TCV Vaccines Market Outlook for Key Countries
Increasing research and development in the U.S.
The U.S. TCV vaccines industry is dominated by large pharmaceutical companies that are continually working to expand their product pipelines. Leading players like Pfizer and Merck drive innovation through strategic acquisitions and partnerships, enhancing their capabilities to develop and market new formulations effectively. This focus on collaboration allows them to leverage advanced technologies and research, ensuring they remain at the forefront of vaccine development and meet the evolving demands of public health.
Government initiatives in China
The China TCV vaccines industry growth is bolstered by government initiatives that provide immunization for priority diseases. Domestic companies have enhanced their production capabilities through collaborations with global pharmaceutical leaders, enabling them to meet rising demand effectively. Domestic companies, such as CNBG and Walvax, are significantly boosting their production capabilities through strategic partnerships with global pharmaceutical leaders. Additionally, the growing emphasis on vaccine innovation in China is supported by a favorable regulatory environment and increased investment in research and development.
Leading vaccine producers in India
India remains a leading vaccine producer due to its extensive manufacturing base, which caters to both domestic and international demand. The Serum Institute of India and Bharat Biotech play crucial roles in ensuring the supply and availability of vaccines, including TCVs, through their large-scale production capabilities and innovative approaches. Their collaboration enhances the region's capacity to combat infectious diseases effectively, contributing significantly to global health initiatives.
Advancements in Japan
The Japan TCV vaccines industry is characterized by a strong acceptance of advanced options, such as combination vaccines. Multinational corporations collaborate with local firms like Takeda and Daiichi Sankyo to introduce and market innovative formulations, enhancing vaccine availability and addressing public health needs effectively. This partnership fosters the development of tailored solutions that meet the specific requirements of the Japanese healthcare landscape, ultimately improving vaccination rates and outcomes in the region.

Get actionable strategies to beat competition: Download Free Sample
Top Strategies Followed by TCV Vaccines Market Players
Emerging Startups in the TCV Vaccines Market
Key Takeaways from Analyst
TCV Vaccines Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 464.9 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.6% | 2032 Value Projection: | USD 1,067.5 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
GSK plc., Sanofi, Bharat Biotech, BioFarma, Zydus Group, Emergent BioSolutions Inc., Bavarian Nordic Inc., Crucell Switzerland LTD., PaxVax, Inc., and BIO-MED |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Driver - Increasing public initiatives for promoting and advancing the development of innovative products
The increasing focus on innovative solutions and new vaccines to combat infectious diseases, particularly typhoid, is driving the TCV vaccines market. Governments and organizations like the World Health Organization are prioritizing vaccine development, recognizing the significant disease burden posed by typhoid in developing regions. Initiatives enhance vaccine access in low- and middle-income countries, supported by regional development centers in Asia and Africa, fostering a robust ecosystem for TCV innovation and distribution.
Market Challenge - Limitations associated with available vaccines
A significant challenge facing the TCV vaccines market is the limitations of existing vaccines. While vaccines for tetanus, diphtheria, and pertussis are available, their efficacy is often short-lived, requiring multiple doses for adequate protection. This can lead to incomplete vaccination schedules if follow-up doses are missed. Additionally, immunity wanes over time, necessitating booster shots. The side effects associated with current vaccines can further hinder compliance, highlighting the urgent need for novel vaccines that offer longer-lasting protection with minimal side effects.
Market Opportunity - Increasing collaboration between pharmaceutical companies and research institutions
The TCV vaccines market is set to experience significant growth due to enhanced collaboration between pharmaceutical companies and research institutions for product development. These partnerships shorten the time required to translate academic research into commercial products by providing funding for early-stage research and facilitating quicker clinical evaluations. This synergy leverages the strengths of both sectors, potentially accelerating the development of more effective TCV vaccines with improved formulations and immunogenicity profiles, driving substantial market expansion.
What goes growth in the global TCV vaccines industry mean for different stakeholders?
The TCV vaccines industry has multiple players with varied designations and offers multiple opportunities based on their scope of operations.
|
Key Pharmaceutical Stakeholder |
Opportunities Due to TCV vaccines Industry Growth |
|
Retail Pharmacies |
Expansion of product offerings to include new drugs and personalized medicine solutions, enhancing customer care and market reach. |
|
Chemical Suppliers |
Growth in demand for specialty chemicals used in drug synthesis, including organic intermediates, catalysts, and reagents. |
|
Pharmaceutical Companies |
Expansion of product pipelines with new drug discoveries, biologics, and biosimilars, capitalizing on growing global healthcare needs. |
|
Contract Research Organizations (CROs) |
Increased outsourcing of clinical trials and drug development, offering opportunities for growth and long-term partnerships. |
|
Contract Manufacturing Organizations (CMOs) |
Growing demand for scalable manufacturing solutions, including biologics production and complex drug formulations. |
|
Diagnostic Equipment Manufacturers |
Expanded markets for diagnostic tools and devices that support personalized medicine and early disease detection. |
|
Healthcare Providers |
New treatment options and innovative therapies, improving patient care and expanding healthcare services. |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients